Clinician Name: Miranda Garland
Clinic Name & Location: Biofeedback Associates of Northeast Florida
Professional Status: Miranda, B.A. is a neurofeedback technician. Chris Branciere, M.S., BCB is a board certified biofeedback therapist, adjunct psychology professor, and PhD student
Abstract
This is a prospective self-report in which vitamin B12 dietary supplements were excluded from a raw, plant-based diet for one year. Vitamin B12 deficiency may result in prolific intracerebral consequences of demyelination, often reflected as frontal and diffuse slowing in the electroencephalogram (EEG). The prevalence of vitamin B12 deficiency is common but consensus on precise cutoff points for plasma vitamin B12 deficiency has been inconsistent. This has raised questions about potential presymptomatic vitamin B12 risks or whether higher vitamin B12 stores could provide additive health benefits. Vitamin B12 values, symptoms potentially associated, methylcobalamin supplementation, and resting EEG recordings before and after neurofeedback (NF) training were examined. Eight sessions of NF training at T3, T4, T5, and T6 resulted in 38% EEG normalization with reduced symptoms of irritability, insomnia, fatigue, and memory problems. Even though blood test results before and after vitamin B12 supplementation readings indicated an increase of 8 mg/mL (from 436 pg/mL to 444 pg/mL), neuroenhancements were not evident without NF training.
Keywords: encephalopathies, methylcobalamin, hematological, individual response specificity, stimulus response specificity, intraindividual variation
Literature Review
Neurological symptoms often precede hematological symptoms that are initially diagnosed based on plasma vitamin B12 analysis (Gröber, Kisters, & Schmidt, 2013). Previous authors have indicated a need to improve diagnostic criteria for vitamin B12 deficiency (Tucker, et al., 2000). If electroencephalography (EEG) biomarkers associated with low vitamin B12 status could be detected earlier - as part of a combination of screening methods to identify subclinical vitamin B12 symptoms, such neurophysiological biomarkers could be used as non-invasive and cost-effective approaches for additional diagnostic value. Neurofeedback (NF) training could also provide early management of preclinical vitamin B12 deficiency. Monitoring EEG activity could serve to identify the degree to which symptoms may be associated with metabolic or psychogenic factors and therefore more or less amenable to change.
Although sample sizes were small in earlier studies that sought to identify signature EEG features related to vitamin B12 deficiency symptoms (Walton, Kiloh, Osselton, & Farrall, 1954; West & Ellis, 1966; Smith, 1962), EEG features among vegan subjects with hypovitaminosis- B12 were interpreted as heterogeneous (vitamin B12 deficiency does generally result in heterogeneous symptoms) and slightly abnormal with diffuse cortical slowing. Vegetarian serum vitamin B12 values were intermediate between controls and vegans, with less EEG abnormality detected. Walton, Kiloh, Osselton, and Farrall (1954) examined EEG features related to vitamin B12 deficiency symptoms of pernicious anemia and plant-based diets; they documented improvements in 79% of post-treatment EEG recordings after oral vitamin B12 supplementation. Slight to moderate excess delta and theta activity were lowered in many subjects and alpha frequency rose within seven days of vitamin B12 treatment. Continued improvements were noted three years after vitamin B12 supplementation in some cases (p. 55). Carmel (2008) also verified symptom reductions beginning within a week of vitamin B12 supplementation.
Smith (1962) speculated, "Although most subjects were clinically normal, the electroencephalographic evidence suggests that occult damage to the central nervous system is taking place and that this form of diet may produce subtle cerebral changes that are unrecognizable by normal clinical methods" (p. 1,658). However, recent studies have not verified this or similar conjectures from Walton, Kiloh, Osselton, and Farrall (1954); and West and Ellis (1966) attributing slight EEG abnormalities to cerebral metabolic adjustments of plant diets (Amodio, et al, 2001; Beezhold, Radnitz & DiMatteo, 2014).
Kaplan and Rosetti (2011) documented diffuse slow EEG activity (5-6 Hz) associated with encephalopathies and they understood much of the research correlating EEG patterns with encephalopathic conditions has been based on case studies so the clinical value of rapidly identifying EEG features associated with encephalopathies has been under-recognized. However, EEG is increasingly used in hospitals for causal and prognostic data. Perhaps EEG recordings can be used to identify encephalopathic symptoms early among those with low vitamin B12 status, too.
Palacios, et al. (2013) noted "clinical severity of vitamin B12 deficiency is often unrelated to B12 concentrations" (p. 1451). The duration of vitamin B12 deficiency is most crucial in determining whether symptoms become irreversible (Healton, 1995). Pacholok and Stuart (2011) stalwartly advocate for substantially higher serum vitamin B12 lower limit cutoff values for the general population: "Serum B12 must be 550 in order to have a good amount of B12 in the CSF.” While this introduces the considerably legitimate topic of neurological symptomology preceding hematological symptoms, what constitutes a good amount of vitamin B12 in CSF is even more unformulated than World Health Organization (WHO) serum vitamin B12 deficiency cutoff values of 203 pg/mL (de Benoist, 2008, p. S242). Klee (2000) revealed that by the same cut-off levels of serum vitamin B12 (>500 pg/mL), there would be inordinately more patients requiring follow-up tests. Although Travica, Ried, Bujnowski, and Sali (2016) logically recognize vitamin B12 requirements vary with age, the authors caution, "when the serum level drops below 500 or 550 pg/mL the cerebrospinal fluid level can become deficient... Some experts suggested the current recommended range of vitamin B12 is too low and that the optimal range should be at least 500–1300 pg/mL" (p. 5). However, the reference of these oft-cited experts was a preliminary study of 14 subjects with senile dementia who had normal ranges of serum vitamin B12 levels (500-1300 pg/mL) and CSF vitamin B12 levels were not related to severity of dementia (Mitsuyama & Kogoh, 1988). Zhang (2016) also noted in a small study that reduced CSF vitamin B12 appeared in subjects with autism and schizophrenia. Generalizing this limited evidence to support increasing serum vitamin B12 cutoffs by such a great margin for the general population demonstrates markedly conservative bias for inclusion of outliers since exhaustive reviews of intake and plasma status of vitamin B12 in different populations has led to the current estimated average daily intake requirements that are not just adequate but increase bodily vitamin B12 stores in healthy people. Lindenbaum, Savage, Stabler, and Allen (1990) evaluated the sensitivity of the 200 pg/mL cutoff value for serum vitamin B12 deficiency in two groups of patients with vitamin B12 deficiency symptoms and arrived at an average false negative result of ~4%. Regland, Abrahamsson, Blennow, Gottfries, and Wallin, (1992) logically furnished rationale for developing a database for different health conditions or populations based not on free serum vitamin B12 levels but ratios of CSF/serum vitamin B12 values.
Subject History
Client Gender: Male
Age: 45
Marital Status: Single
Socioeconomic status: Middle Class
Public or Private School: Grew up attending public and Catholic Sunday School
Years in School: Current PhD student
Started Dating at Age: 16
Number of Friends: 4-6 close friends
Current social Groups: Soccer team
Dating Frequency: None
Sports Played: Soccer and Surfing
Exercise Frequency: 2 Days/Week
Parental Status: Mother recently passed away from heart condition
Sexual or Physical Abuse: None
Personal substance abuse: None. No Alcohol, Caffeine, Herbal Remedies, or Medication.
Family Substance Abuse: Smoking-one parent.
History of Head Trauma: None.
Presenting Symptoms
Insomnia: Sleep onset
Attention: Low attention
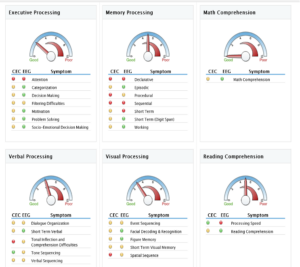
Reading comprehension: Slow processing speed while reading
Memory: CPTs show Declarative Memory, Sequential Memory, and Short Term Memory Problems
Headaches: None
Rumination: Frequent
Worry: Low
Suicidal Thoughts: None
Mood: Irritable, angry, inhibited, and busy after mother passed, particularly following missed meals. Insomnia, irritability, fatigue, and memory problems were among the symptoms identified in the first qEEG summary report and coincidentally, these are common symptoms known to be associated with vitamin B12 deficiency (Audebert, Gendre, & Le Quintrec, 1978; Dror, & Allen, 2008; Briani, et al., 2013).
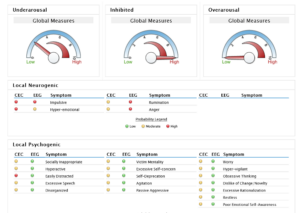
Metabolic Issues
Metabolic issues were not apparent.
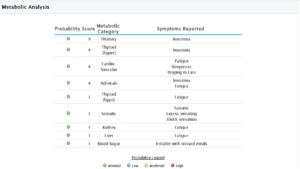
Metabolic Analysis
Training
I subscribed to topographic mapping of quantitative electroencephalography (qEEG) using Brainmaster Discovery 24e hardware. A second brain map was completed for comparison to the baseline eyes open and eyes closed qEEG brain maps, following an 8-session (40 minutes each) massed practice schedule using BrainDX normative database and Brainmaster Atlantis I hardware for four channels of EEG z-score NF training. The training sites were the four most statistically deviant sites (T3, T4, P4, and O1 using the International 10-20 System for standardized electrode placement) from the eyes closed primary protocol based upon NewMind qEEG analysis. When the threshold for training criteria was achieved approximately 80% of the time, the threshold level was raised one percent. After cessation of NF training, eight oral doses of vegan methylcobalim (2.4 µg/day) were consumed in accordance with WHO daily vitamin B12 requirements for adults (FAO, 2001, p. 69; de Benoist, 2008, p. S242). A final brain map following eight sessions of vitamin B12 supplementation allowed comparisons between the baseline neurometric recordings, the first intervention (NF training), and the second intervention (oral methylcobalim supplementation).
Pre Post Trend Screen Protocol 1
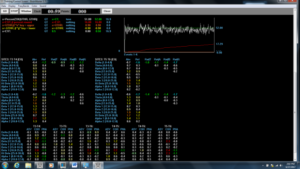
Figure 1. Z-score training session 1 at T3/T4 and T5/T6 5-minutes and 59 seconds into training. The threshold of 48% was initially set to meet age and gender-specific criteria within 0.9 standard deviations above and below normative values approximately 70 - 80% of the training time and that was the threshold setting during the training. For comparison purposes, screenshots for 52% threshold settings are displayed here.
Pre Post Trend Screen Protocol 2
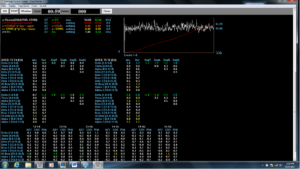
Figure 2. Z-score training session 8 at T3/T4 and T5/T6 during the 5:59 mark. The training threshold was gradually increased to 52% by the 5th training session to meet the age and gender-specific criteria within 0.9 standard deviations above and below normative values approximately 70% or more of the training session.
Pre Post Map
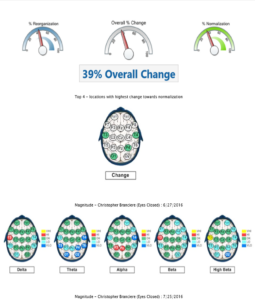
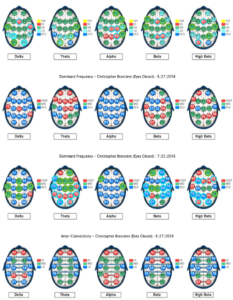
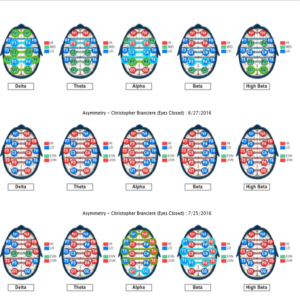
Figure 3. Baseline qEEG compared to qEEG following neurofeedback training
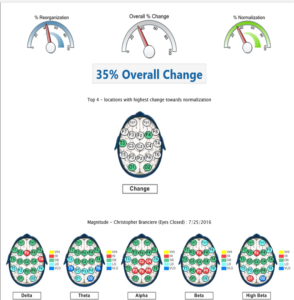
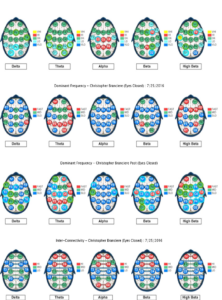
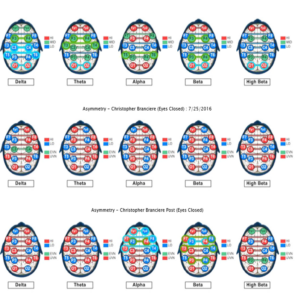
Figure 4. Baseline qEEG compared to qEEG following both neurofeedback training and oral vitamin B12 intake interventions.
Results
The most dramatic differences were noticed between baseline and post-neurofeedback training in eyes closed maps. T3 and T4 are the nearest 10/20 cortical sites to the hippocampus and amygdala and training these sites seemed to reduce irritability and memory problems (common vitamin B12 deficiency symptoms) indicated as problems in the baseline brain map. No evidence was found for neuroenhancements by increasing low normal serum vitamin B12 values 8 pg/mL (from 436 pg/mL to 444 pg/mL) with oral vitamin B12 supplementation. A slightly disproportionate increase in serum vitamin B12 exceeded what would be proportionately expected for eight consecutive days of 2.4 µg/d oral methylcobalim supplementation. A spillover effect or delayed changes from NF training could have occurred following cessation of NF training but the overall percent change in the maps between the baseline and post vitamin B12 supplementation was less than would be expected, given the 39% overall change and higher percent of normalization that occurred between the baseline and post NF training. The fact that the NF training was only eight sessions and was not a spaced practice schedule may have reduced generalization, though. Higher reorganization relative to normalization between the post NF training and post vitamin B12 supplementation maps was observed. Subjective awareness of improved reading comprehension and processing speed were also noticed following NF training.
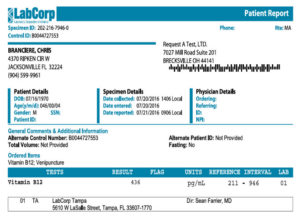
Figure 5. Baseline serum vitamin B12
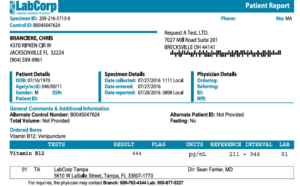
Figure 6. Post-intervention serum vitamin B12
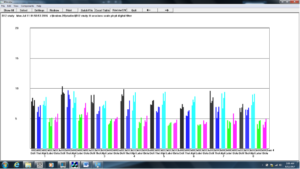
Figure 7. Z-score training sessions (1-8)
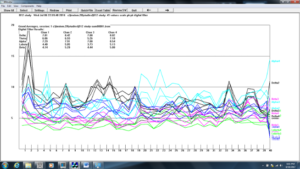
Figure 8. Z-score training session #1
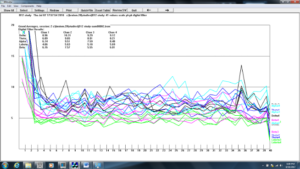
Figure 9. Z-score training session #2
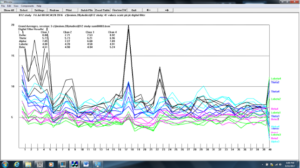
Figure 10. Z-score training session #3
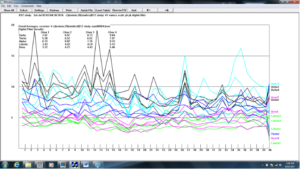
Figure 11. Z-score training session #4
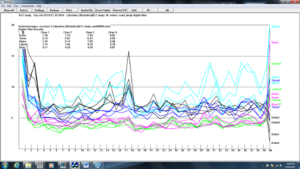
Figure 12. Z-score training session #5
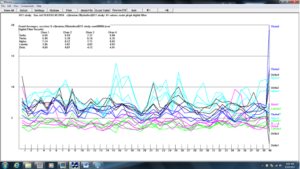
Figure 13. Z-score training session #6
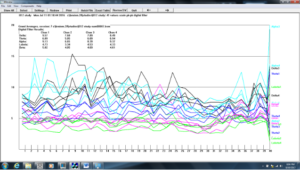
Figure 14. Z-score training session #7
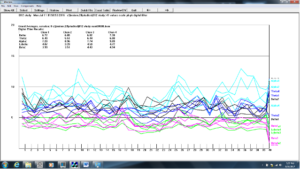
Figure 15. Z-score training session #8
Discussion
Vitamin B12 deficiency and demyelination are often reflected as diffuse slowing in the EEG. Vitamin B12 deficiency is caused by malnutrition, malabsorption, or reabsorption problems and may be comorbid or secondary to digestive disorders such as reduced colonic bacteria from vitamin D or iron deficiency, alcohol intake, diabetes, etc. (Laufer, 2004; Carmel, 2008; Solomon, 2011; Gominak, 2015). Smoking, medications, and cooking food can also reduce absorption or deplete vitamin B12 (Linnell, Smith, Smith, Wilson, & Matthews, 1968; Nishioka, Kanosue, Yabuta, & Watanabe, 2011; “Nutritional Effects of Food Processing,” 2014). Increased vitamin A and vitamin C intake will increase vitamin B12 absorption (“Mayo Clinic,” n.d.). Cyanocobalamin B12 intake can restore serum vitamin B12 values and it is a more common and cost-effective supplement form but less bioavailable than methylcobalim B12, which is premethylated (Adams, 2011). There is a lack of evidence demonstrating detrimental effects of the small amounts of cyanide in the cyanocobalamin B12, so far (West & Ellis, 1966).
Wide-ranging diagnoses with differential effects such as vitamin B12 deficiency are traditionally categorized as diverse sets of fluctuating and overlapping symptoms, such as fatigue, insomnia, mood, or memory problems. Such heterogeneous symptoms are not likely to be unambiguously reflected in EEG as distinctly clear categories of metabolic abnormalities, particularly in early stages. Identifying EEG features potentially associated with individual symptoms instead of signature feature patterns or broad diagnostic categories may be a more fruitful approach to addressing metabolic problems such as vitamin B12 deficiency. EEG activity reflects numerous intraindividual variations (different individual responses to the same stimuli at different times), stimulus response specificity (products of evoking stimuli), and individual response specificity (unique products of responding individuals, i.e., some subjects show more hematological symptoms while others exhibit more neurological symptoms). A long-term follow-up self-report is warranted to estimate vitamin B12 absorption and reabsorption rates by monitoring serum vitamin B12 over time and controlling oral vitamin B12 intake with continued exclusion of digestive compromises. Future studies are necessary to clarify the degree to which EEG activity may correlate with neurological symptoms of B12 hypovitaminosis and to determine whether diffuse slowing or other, as yet unrecognized, neurological biomarkers can be detected before the threshold effect of vitamin B12 deficiency.
Conclusions
Spaced NF training sessions, as opposed to mass training, may increase generalization. NF training could potentially mask chronic deficiencies if it can be used to manage metabolic symptoms. Diffuse slowing seems to be the most common sign of demyelination and low vitamin B12 status that has been detected in the EEG so far, but the literature on the topic is anemic and the symptoms of vitamin B12 deficiency are diverse. Oral supplementation (particularly with methylcobalimin) can quickly increase serum vitamin B12 levels if absorption problems are not an issue.
Vitamin B12 is necessary for maintaining myelin sheath and neurotransmitter synthesis (Herrmann & Obeid, 2008). The prevalence of vitamin B12 deficiency is common and reported to range vastly between 6-40% (Palacios, 2013). Dietary vitamin B12 has low bioavailability and it becomes more difficult to absorb with age but most of it is reabsorbed by the ileum in healthy adults. Carmel (2008) documented fractional vitamin B12 absorption decreases with intake but total vitamin B12 absorption increases with vitamin B12 intake. Oral doses of vitamin B12 ranging from 1 μg – 1000 μg correspond with absorption rates of decreasing efficiency from 56% - 1.3%. These figures serve as critical reminders that frequent intake of relatively low vitamin B12 doses (as with many other nutrients) is far more efficiently absorbed and the otherwise overworked phrase “eat a balanced diet” becomes meaningfully applicable in light of this ratio data (Carmel, 2008; Tucker, et al., 2000). Vitamin B12 intake is also more efficient on an empty stomach. One may exhibit statistically normal serum vitamin B12 and still develop functional vitamin B12 deficiency, particularly among infants who have not had time to build up stores, those with diabetes, and the elderly, in which various types of dementia frequently occur with associated low levels of vitamin B12 in CSF (Dror, & Allen, 2008; Solomon, 2011). Paradoxically, one may exhibit low serum vitamin B12 without symptoms, depending on how long vitamin B12 was stored in the kidney, liver, and other cells (Bopape, Mbhenyane & Alberts, 2008, p. 334).
Vitamin B12 deficiency symptoms may begin before exhausting bodily stores or by excluding dietary vitamin B12 for an average of 5-6 years, depending on age, digestive cofactors, etc. (Bopape, Mbhenyane & Alberts, 2008, p. 334). It may be tempting to presume saturation of vitamin B12 stores could provide incremental life promoting benefits or that monotonic trends of increased vitamin B12 stores could create health protective effects up to or beyond serum vitamin B12 levels of 500 pg/mL. Prospective studies on vitamin B12 status and diseases of affluence have yielded correlations with breast cancer risk slightly elevated among those with serum vitamin B12 up to 280 pg/mL (Wu, et al., 1999). Reduced plasma homocysteine levels were also associated with serum vitamin B12 levels up to 300 pmol/l and elevated homocysteine levels may increase cardiovascular disease risk (Fenech, Aitken, & Rinaldi, 1998, p. 1163; Ryan-Harshman, & Aldoori, 2008, p. 536; “Linus Pauling Institute,” 2015). The most convincing support for raising vitamin B12 lower threshold cutoff levels applies to reduced risks of neural tube defects with serum vitamin B12 levels ≥400 ng/L (Molloy, 2009, p. 918). But WHO already advised higher vitamin B12 (+40%) intake for pregnancy and the recommended daily dose of 2.4 µg/day for most adults (who have a 50% reabsorption rate) provides 0.4 µg/day beyond daily requirements (Ladipo, 2000, p. 284; Joint, F. A. O., & World Health Organization, 2005). Unless clinically deficient, there is a paucity of evidence to support increasing vitamin B12 stores for healthy adults who want to enhance cognition or that increased vitamin B12 intake has protective effects for dementia (Malouf & Areosa Sastre, 2003; Health Quality Ontario, 2013, p. 1).
References
Amodio, P., Caregaro, L., Pettenó, E., Marcon, M., DelPiccolo, F., & Gatta, A. (2001). Vegetarian diets in hepatic encephalopathy: facts or fantasies? [PDF file]. .Digestive and Liver Disease, 33(6), 492-500. http://ac.els-cdn.com/S1590865801800281/1-s2.0-S1590865801800281-main.pdf?tid=0aea4e30-4c49-11e6-9e6a-00000aab0f26&acdnat=1468778908_5bd3b0835957d1ecd36087bedadb5513
Audebert, M., Gendre, J. P., & Le Quintrec, Y. (1978). [Folate and the nervous system (author's transl)]. La semaine des hopitaux: organe fonde par l'Association d'enseignement medical des hopitaux de Paris, 55(31-32),1383-1387.
Adams, M. (2011). Vitamin B-12 warning: Avoid cyanocobalamin, take only methylcobalamin. Natural News. http://www.naturalnews.com/032766_cyanocobalamin_vitamin_B-12.html#ixzz4EWTZs9XP
Beezhold, B., Radnitz, C., & DiMatteo, J. (2014). Large vegan sample reports less anxiety and stress than omnivores (823.3). The FASEB Journal,28(1 Supplement), 823-3. http://www.fasebj.org/content/28/1_Supplement/823.3.short
Bopape, M. M., Mbhenyane, X. G., & Alberts, M. (2008). The prevalence of anaemia and selectedmicronutrientstatus in pregnant teenagers of Polokwane Municipality in the LimpopoProvince. South African Journal of Clinical Nutrition, 21(4), 332336.http://www.tandfonline.com/doi/abs/10.1080/16070658.2008.11734175
Briani, C., Dalla Torre, C., Citton, V., Manara, R., Pompanin, S., Binotto, G., & Adami, F. (2013). Cobalamindeficiency: clinical picture and radiological findings. Nutrients, 5(11), 4521-4539.http://www.mdpi.com/2072-6643/5/11/4521/htm
Carmel, R. (2008). How I treat cobalamin (vitamin B12) deficiency. Blood,112(6), 2214-2221.http://www.bloodjournal.org/content/112/6/2214 de Benoist, B. (2008). Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. [PDFfile]. Food and nutrition bulletin, 29(2 Suppl), S238. http://www.who.int/nutrition/publications/micronutrients/FNBvol29N2supjun08.pdf
Dror, D. K. & Allen, L. H. (2008). Effect of vitamin B12 deficiency on neurodevelopment in infants: currentknowledge and possible mechanisms. [PDF file]. Nutrition reviews, 66(5), 250 – 255. http://naldc.nal.usda.gov/download/17955/PDF
FAO, W. (2001). Human Vitamin and Mineral Requirements. Report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand. [PDF file]. Food and Nutrition Division, FAO Rome. http://www.fao.org/3/a-y2809e.pdf
Fenech M, Aitken C & Rinaldi J (1998): Folate, vitamin B12, homocysteine status and DNA damage in young Australian adults. Carcinogenesis 19, 1163–1171. http://carcin.oxfordjournals.org/content/19/7/1163
Gominak, S. (2015). What does D hormone deficiency look like? Vitamin D Hormone.drgominak.com/vitamin-d-hormone.html
Gröber, U., Kisters, K., & Schmidt, J. (2013). Neuroenhancement with vitamin B12—underestimated neurological significance. Nutrients, 5(12), 5031-5045. http://www.mdpi.com/2072-6643/5/12/5031/htm
Health Quality Ontario. (2013). Vitamin B12 and Cognitive Function: An Evidence-Based Analysis. Ontario Health Technology Assessment Series,13(23), 1–45. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3874776/
Herrmann, W., & Obeid, R. (2008). Causes and early diagnosis of vitamin B12 deficiency. [PDF file]. Dtsch Arztebl Int, 105(40), 680-5. http://www.homocysteine-panel.org/website_en/pdfdocs/m680.pdf
Joint, F. A. O., & World Health Organization. (2005). Vitamin and mineral requirements in human nutrition. [PDF file]. http://apps.who.int/iris/bitstream/10665/42716/1/9241546123.pdf
Kaplan, P. W., & Rossetti, A. O. (2011). EEG patterns and imaging correlations in encephalopathy: encephalopathy part II. Journal of Clinical Neurophysiology, 28(3), 233-251. https://www.researchgate.net/publication/51184533_EEG_Patterns_and_Imaging_Correlations_in_Encephalopathy_Encephalopathy_Part_II
Klee, G. G. (2000). Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. [PDF file]. Clinical chemistry, 46(8), 1277-1283.http://www.clinchem.org/content/46/8/1277.full.pdf+html
Ladipo, O. A. (2000). Nutrition in pregnancy: mineral and vitamin supplements. The American journal of clinical nutrition, 72(1), 280s-290s. http://ajcn.nutrition.org/content/72/1/280s.full
Laufer, E. M., Hartman, T. J., Baer, D. J., Gunter, E. W., Dorgan, J. F., Campbell, W. S., ... & Taylor, P. R. (2004). Effects of moderate alcohol consumption on folate and vitamin B12 status in postmenopausal women. European journal of clinical nutrition, 58(11), 1518-1524.http://www.nature.com/ejcn/journal/v58/n11/full/1602002a.html
Linnell, J. C., Smith, A. D., Smith, C. L., Wilson, J., Matthews, D. M. (1968). Effects of smoking on metabolism and excretion of vitamin B12. British Medical Journal.;2(5599):215-216.http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1985886/
“Linus Pauling Institute.” (2015). Vitamin B12. Micronutrient Information Center.http://lpi.oregonstate.edu/mic/vitamins/vitamin-B12
Malouf, R., & Areosa Sastre, A. (2003). Vitamin B12 for cognition. The Cochrane Library.http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004394/full
“Mayo Clinic.” (n.d.). Mayo Medical Laboratories. Test ID: B12 Vitamin B12 Assay, Serum.http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/9154
Mitsuyama, Y., & Kogoh, H. (1988). Serum and Cerebrospinal Fluid Vitamin B12 Levels in Demented
Patients with CH3—B12 Treatment—Preliminary Study—. Psychiatry and Clinical
Neurosciences, 42(1), 65-71. http://onlinelibrary.wiley.com/doi/10.1111/j.1440-1819.1988.tb01957.x/abstract
Molloy, A. M., Kirke, P. N., Troendle, J. F., Burke, H., Sutton, M., Brody, L. C., ... & Mills, J. L. (2009). Maternalvitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics, 123(3), 917-923. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4161975/
Nishioka, M., Kanosue, F., Yabuta, Y., & Watanabe, F. (2011). Loss of vitamin B 12 in fish (round herring)meats during various cooking treatments.Journal of nutritional science and vitaminology, 57(6),432-436. https://www.jstage.jst.go.jp/article/jnsv/57/6/57_6_432/_article
Palacios G, Sola R, Barrios L, Pietrzik K, Castillo MJ, González-Gross M. (2013) Algorithm for early diagnosisof vitamin B12 in elderly people. [PDF file]. Nutr Hosp 2013;28:1447–52.http://www.nutricionhospitalaria.com/pdf/6821.pdf
“Nutritional Effects of Food Processing.” (2014). SelfNutritionData. http://nutritiondata.self.com/topics/processing
Ryan-Harshman, M., & Aldoori, W. (2008). Vitamin B12 and health. Canadian Family Physician, 54(4), 536-541.http://www.cfp.ca/content/54/4/536.full
Regland, B., Abrahamsson, L., Blennow, K., Gottfries, C. G. and Wallin, A. (1992), Vitamin B12 in CSF: reduced CSF/serum B12ratio in demented men. Acta Neurologica Scandinavica, 85: 276–281. doi:10.1111/j.1600-0404.1992.tb04044.x
Smith, A. D. M. (1962). Veganism: a clinical survey with observations on vitamin-B12 metabolism. British medical journal, 1(5293), 1655. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1958824/
Solomon, L. R. (2011). Diabetes as a cause of clinically significant functional cobalamin deficiency. Diabetes Care,34(5), 1077-1080. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3114505/
Travica, N., Ried, K., Bujnowski, R., & Sali, A. (2016). Integrative health check reveals suboptimal levelsin a number of vital biomarkers. Advances in Integrative Medicine. https://scholar.googleusercontent.com/scholarq=cache:e4Bf5cbLHlEJ:scholar.google.com/+Serum+and+cerebrospinal+fluid+vitamin+B12+levels+550&hl=en&as_sdt=0,10&as_ylo=2012
Tucker, K. L., Rich, S., Rosenberg, I., Jacques, P., Dallal, G., Wilson, P. W. F., & Selhub, J. (2000). Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring Study. Am J Clin Nutr 71 (2). pp 514-522. http://m.ajcn.nutrition.org/content/71/2/514.long
Walton, J. N., Kiloh, L. G., Osselton, J. W., & Farrall, J. (1954). The electroencephalogram in pernicious anaemiaand subacute combined degeneration of the cord. Electroencephalography and clinical neurophysiology, 6, 45-64. http://www.sciencedirect.com/science/article/pii/0013469454900061
Zhang, Y., Hodgson, N. W., Trivedi, M. S., Abdolmaleky, H. M., Fournier, M., Cuenod, M., ... & Deth, R. C.(2016). Decreased brain levels of vitamin B12 in aging, autism and schizophrenia. [PDF file]. PloS one, 11(1), e0146797. http://journals.plos.org/plosone/article/asset?id=10.1371/journal.pone.0146797.PDF
West, E. D. & Ellis, F. R. (1966). The electroencephalogram in veganism, vegetarianism, vitamin B12 deficiency, and in controls. Journal of Neurology, Neurosurgery & Psychiatry, 29(5), 391-397.https://scholar.google.com/scholar?hl=en&q=+The+electroencephalogram+in+veganism%2C+vegetarianism%2C+vitamin+B12+deficiency%2C+and+in+controls&btnG=&as_sdt=1%2C10&as_sdtp=
Wu K, Helzlsouer KJ, Comstock GW, Hoffman SC, Nadeau MR & Selhub J (1999) A prospective study on folate, B12, and pyridoxal 5'-phosphate (B6) and breast cancer. Cancer Epidemiol. Biomarkers Prev. 8, 209–217.http://cebp.aacrjournals.org/content/8/3/209.long