Time-Limited Neurofeedback Training at Cz Facilitates Sensorimotor Recovery Following Spinal Cord Injury: A Case Study
Robert E. Longo, MRC, LPC, BCN, Serendipity, Lexington, NC
John T. Hummer, Ph.D., BCN, James H. Quillen V.A. Hospital, Johnson City, TN
Abstract
There will be occasions when the informed neurofeedback practitioner will opt to use an alternate training protocol, rather than a protocol derived from analysis of QEEG data. Deviation from standard procedure should be guided by reasoned a-priori considerations (e.g. knowledge of relevant research, understanding of brain-behavior relationships, anecdotal information from trusted colleagues, personal experience with a particular protocol, client response, etc.), as well as by clinical judgement and intuition. This case study reviews the successful use of time-limited neurofeedback with a fifty-eight year-old female who presented with complications of recent spinal surgery, including loss of sensation in both legs, and inability to walk. It was hypothesized that uptraining of Sensorimotor Rhythm (SMR) at site Cz, with inhibits on excessive slow wave activity (e.g. Theta) and excessive fast wave activity (e.g. Beta and/or High Beta) would safely and efficiently facilitate improvements in sensation, movement, and mood-related symptoms. Asymmetry analysis suggested physiological symptoms of depression and anxiety, despite the client’s unawareness of distressing moods. The client reported improvement in sensation and motor response following the very first session, conducted prior to the formal QEEG assessment. Progressive improvements were reported almost weekly over the next fourteen sessions. The client regained sensation incrementally, accompanied by co-awareness of emerging physical pain. Before long, she reported being able to able to stand and walk, first the aid of a walker, and then with a cane. By the fifteenth session, she reported being able to walk without the need of a cane. She also reported relief of lifelong migraine headaches, which had resurfaced during the latter sessions. She opted to discontinue neurofeedback, eager to get back to her busy life, which included travel. Commenting on her neurofeedback experience, she exclaimed, “I have made more gains than any of the medical doctors ever gave me hope for!”
Our findings replicate those of existing research demonstrating the robust effectiveness of SMR training at Cz. That research can now be extended to include facilitation of sensorimotor improvement after recent spinal cord injury. Because of the incredibly complex interconnected neural circuitry throughout the Central Nervous System, together with the brain’s capacity to functionally reorganize neural networks under optimal conditions in response to injury occurring anywhere within the CNS, even a simple and straightforward protocol (such as SMR training at Cz) remains a powerful tool with which to facilitate neuroplastic recovery processes.
Introduction
QEEG acquisition and statistical data analysis forms that basis of standard neurofeedback assessment and protocol selection. A competently- acquired QEEG assessment provides valuable information about the current functional status of the client’s brain, identifies 10-20 sites that statistically deviate from the population norm (to inform intervention protocols), identifies factors that may impact the potential effectiveness of neuro-therapeutic intervention, and can serve as a physiological baseline from which to assess future change. Change(s) in the QEEG may reflect the effects of active interventions, or may reflect exposure to adverse conditions (e.g. increased life stress, traumatic or neurologic injury, exposure to neurotoxins, changes in metabolic states of the body, etc.)
There are occasions when the informed practitioner selects a protocol based upon a-priori knowledge (data acquired through training and experience). Whether stated explicitly or reasoned intuitively, the underlying clinical hypothesis or rationale holds that the alternate protocol will accomplish treatment goals both safely and efficiently (possibly more efficiently than the statistically-derived protocol(s). Factors guiding protocol selection may include results of published and unpublished treatment research, anecdotal information from respected colleagues, previous success with a particular protocol, client characteristics and goals, and familiarity with neuroscience research furthering our understanding of brain-behavior relationships, including the neural underpinnings of neurologic and psychiatric symptoms.
Literature Review
The following review will attempt to integrate two lines of inquiry. First, the focus will be upon published studies exploring neurofeedback’s effectiveness in facilitating improvements in sensory and/or motor functioning and/or neuropathic pain reduction, in cases of spinal cord injury involving sensory/motor dysfunction or paralysis. Secondly, published studies will be reviewed that have specifically utilized one-channel SMR uptraining at site Cz to address symptom manifestations of somatic dysfunction, such as fibromyalgia pain, and particular forms of neuropathic pain (which involve a combination of sensory deficits, and pain). To date, however, there are no known studies of one-channel training that have addressed sensory/ motor dysfunction related to spinal cord injury. This case study will attempt to begin to fill this gap in the existing literature.
Overview: Training at Site Cz
Historically, there is ample evidence supporting the effectiveness of operant conditioning of brainwave activity at site Cz (e.g. uptraining the amplitude of Sensorimotor Rhythm or SMR). SMR was first identified by Barry Sterman, and refers to normal activity in the 13-15 Hz range over the motor cortex (motor strip) associated with a calm, motionless state of readiness. One channel SMR uptraining at Cz was applied by several researchers and clinicians to address a variety of clinical symptoms and problems (for reviews, see Egner & Gruzalier, 2004; Othmer, 2016.) A comprehensive listing of symptoms/conditions for which one-channel training at Cz has been found effective is presented in Soutar & Longo (2011), Tables 10 through 17.
Site Cz on the scalp overlies the midpoint of the Central Sulcus, the large crevice that partly separates the two cerebral hemispheres. Each EEG sensor (when placed at an International 10-20 system site) is estimated to sample hundreds of thousands of dipole layers (each dipole layer containing about 600,000 macro-columns or 60,000 pyramidal neurons.) The surface area covered by each sensor spans a cortical surface area of approximately 6 squared centimeters, roughly fitting within the circumference of a silver dollar coin (Nunez & Srinivasan, 2006.) Of the electrical activity sampled by a scalp sensor (amplitude is measured in microvolts), approximately 60% corresponds to localized network activity (source generators) and 40% reflects volume conduction from activity in distal areas of the brain, as well as from random sources (Nunez &, Srinivasan, 2006).
Site Cz is also thought to overlie portions of the sensory and motor homunculi of each hemisphere, namely the sensory and motor areas corresponding to the leg, foot, toes (and deeper down into the Central Sulcus, the genitalia). The ‘sensorimotor strip’ of each hemisphere maps the sensorimotor functions occurring on the contralateral side of the body. By increasing activation of central (and adjacent homologous) areas of the sensorimotor strip of both hemispheres simultaneously, effective communication across the corpus callosum linking those areas should be increased, hypothetically.
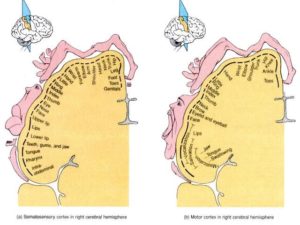
As a guide to the clinician conducting amplitude training at Cz, published normative data for this site (e.g. amplitudes; standard deviations; minimum vs. maximum ranges for Theta, Alpha, Beta, and SMR, in the eyes closed vs. eyes open conditions), were published by Montgomery, Robb, Dwyer & Gontkovsky (1998), based upon a sample of 49 healthy young adults.
To further guide amplitude training along midline sites (along the Central Sulcus), Siegfried and Susan Othmer have identified optimal reward frequency values (in Hz) for the frontal-midline (Fz), meso-midline (Cz), and parietal-midline (Pz) sites. Frontal midline training (Fz) optimizes at about 2 Hz lower than at Cz (hence, the optimal training frequencies are 12 Hz at Fz, and 14 Hz at Cz). Parietal training (Pz) optimizes at about 4 Hz lower than at Cz (hence, the optimal training frequencies are 10 Hz at Pz, and 14 Hz at Cz). Training of amplitude is recommended using frequency bandwidths spanning 3 Hz (Othmer and Othmer).
Neurofeedback studies (operant conditioning of brainwave activity) targeting sensory-motor dysfunction and associated neuropathic pain
In a case study, Sime (2004) used biofeedback and neurofeedback to reduce symptoms of chronic pain and bruxism associated with Trigeminal Neuralgia in a 46 year-old woman. Sensors were placed at sites T4, C3, C4, C3-C4, and T3-T4 (sensorimotor strip, or anterior temporal areas). A total of 29 neurofeedback and 10 biofeedback sessions were conducted over 37 weeks, involving uptraining of Low Alpha (7.5 to 10.5 Hz), with inhibits on 2 – 7 Hz (Delta-Theta) and 22-30 Hz (High Beta) activity. The author reported that up-training of Alpha at T3 and T4 resulted in the most immediate pain reduction. The client decided to cancel planned surgery (to sever the Trigeminal Nerve) and was able to discontinue pain medications, with benefits (continued pain relief) maintained at a 13 months follow-up.
Jensen, Grierson, Tracy-Smith, Bacigalupi, and Othmer (2007) reported outcome data on 18 clients with Complex Regional Pain Syndrome, Type I, who took part in an intensive multi-disciplinary training program (20 days in length; 5-6 hours of active interventions per day.) Multimodal interventions included medication management, physical therapy, psychotherapy, and neurofeedback (30 minute sessions). Single-channel bipolar montages were used (Active site-Reference site, both on scalp) at the following site combinations: P3-P4, Fp1-Fp2, T3-T4, FPO2-A2, Cz-Fz, F7-F8, and F3-F4. (FPO2 is located on the nasal-side upper corner of the right orbit, thought to sample right amydalae activity; A2 is the right ear, used as the reference sensor). Rewards and inhibits were placed on activity ranging from 0.5 to 30 Hz (Delta through High Beta), with choices of rewards/inhibits having been individualized for each client (which makes study methodology virtually impossible to replicate). The authors reported that all clients reported significant reduction of pain intensity at primary (bodily) pain sites (50% site-specific reduction), with half of the participants reporting at least a 30% overall reduction of pain (Jensen, et. al., 2007).
Ibric & Dragomirescu (2009) treated 74 clients with chronic pain (related to multiple etiologies) that had been unresponsive to traditional medical care. All 74 clients completed 19 or more neurofeedback sessions (which incorporated training at Cz in some instances). A total of 68 of 74 clients (92%) reported clinically significant improvements in sensory and/or motor functioning, and pain reduction. The authors reviewed ten cases in detail, spanning several neuropathological conditions and associated comorbid conditions. The examples included cases of reflex sympathetic dystrophy, spasticity post-meningitis, spinal cord injury, idiopathic neuropathy, neuropathy post-laminectomy, rheumatoid arthritis, and Parkinson’s Disease. Pre-QEEG to Post-QEEG changes, cognitive performance data, and graphs tracking session-to-session progress, were illustrated. Each client served as his/her own control, pretreatment to posttreatment. The primary study limitations included sample selection bias, and lack of a sham neurofeedback control group (omitted most likely because of practical and ethical concerns, as the research occurred in a Rehabilitation Facility where active intervention was the primary purpose). One could argue that these were desperate and very motivated clients (not necessarily representative of all clients with similar conditions), and that placebo (positive expectancy) effects could have impacted client motivation and intensity of rehabilitation effort. A strength of the study was the inclusion of heterogenous conditions, all of which benefited from neurofeedback, attesting to its robustness.
Hassan, Fraser, Conway, Allan, & Vuckovic (2016) provided neurofeedback to 7 clients diagnosed with paraplegia (with associated central neuropathic pain of the legs. The neurofeedback protocol (40 or more sessions) targeted sensorimotor strip sites C3, Cz, and C4, along with right parietal site P4. A reward was placed on the Alpha bandwidth (9 – 12 Hz), while inhibits were placed on Theta activity (4 – 8 Hz) and High Beta activity (20-30 Hz). Four protocols were used: (1) Uptraining SRM with inhibits on Theta and High Beta at Cz; (2) Uptraining of Alpha with inhibits on Theta and High Beta at P4; (3) Uptraining of Alpha with inhibits on Theta and High Beta at C3; and (4) Uptraining of Alpha with inhibits on Theta and High Beta at C4. A placebo (sham neurofeedback) control condition was also included, whereby subjects viewed a training screen with images taken previously during their own uptraining of Alpha amplitude at Oz (an occipital site that is not associated with pain generation). A total of 4/7 patients completed 40 sessions of neurofeedback (in addition, 1 individual completed 20 sessions.) The sessions entailed roughly 50 minutes of training: 2 minutes of baseline at the start and end of each session; then training in at least two of the above conditions, using auditory feedback 20% – 30% of the time and visual reinforcement 70% – 80% of the time. Overall, 4/5 clients reported a clinically significant reduction of pain (>30 % pain reduction) which lasted at least a month beyond the intervention. QEEG changes from pre-treatment to post-treatment were noteworthy for a reduction of power in the Beta range, with changes most apparent in the Dorsolateral Prefrontal Cortex, Anterior Cingulate Cortex, and Insular Cortex. The Hassan et. al. research was a naturalistic study of paraplegic patients, and incorporated standard protocols and a ‘sham’ protocol that all clients were exposed to, to an equivalent degree. The primary limitations of the study are the small sample size (placing constraints on the type and reliability of statistical analyses performed in data analysis) and possible sampling/selection bias (only motivated, treatment-seeking individuals were included). It is quite difficult, however, to retain partipants in a study this long and involved without strong incentives, whether internal (‘I want to get better’) or external (‘I am being paid for my participation.’) This study was apparently conducted without the aid of a research grant.
Jensen, Gertz, Kupper, Braden, Howe, Hakimian, & Sherlin (2013) studied the effectiveness of neurofeedback intervention in a sample of 10 clients with spinal cord injury and chronic pain. Citing insufficient evidence of superiority of any one neurofeedback protocol over another (in ameliorating chronic pain), the authors included three commonly-used protocols. Reviewing previous research, the authors noted that some studies used sensor placements at T3 and T4 (temporal, ventral stream sites near the primary sensory cortex), whereas, other studies used a single active sensor placement at Cz (the midline of the sensorimotor cortex), while yet other studies trained at parietal sites (e.g. P3, Pz, P4). The parietal cortex is involved in the processing and integration of sensory stimuli, and thought to play a role in central sensitization. Jensen, et. al. further noted that previous studies identified (1) excessively high amplitudes of fast wave activity (e.g. Beta or High Beta) and/or (2) excessive slow wave activity (Theta). The authors chose to place inhibits on both of these frequency bands in their study. Jensen, et. al. further noted that some studies uptrained (rewarded) the amplitude of SMR (13 –15Hz), which they, too, incorporated into their study. They conceded that, in practice, choice of protocol is likely influenced by a combination of factors – and unlikely to be based solely on pre-selected 10-20 sites, popular bandwidth rewards and inhibits, or symptom profile alone.
Although a control group (i.e. no treatment, treatment as usual, or sham neurofeedback control) was not included in Jensen et. al. study, the authors performed baseline and post-treatment outcome measures. They trained all participants with three protocols described below, and counterbalanced the protocol order by randomly assigning the sequence. Four consecutive sessions were conducted with each protocol. The protocols that were incorporated into the study consisted of: (A) uptraining of Alpha amplitude while inhibiting Beta amplitude at sites T3 and T4; (B) uptraining the amplitude of 10-15 Hz activity (Alpha-SMR) while inhibiting amplitudes of Beta and Theta at C3-A1 and C4-A2 (A1 is the left ear; A2 is the right ear); and (C) uptraining the amplitude of 10-15 Hz (Alpha/SMR) activity while inhibiting amplitudes of Beta and Theta at P3-A1 and P4-A2.
Following the 12 sessions of neurofeedback, 70% of patients reported ‘some’ pre-to-post reduction in ‘worst pain intensity’ and ‘pain unpleasantness’, and this finding maintained at the 3-month follow-up. One patient reported a clinically significant pain reduction (more than 30% decrease from pre-intervention to post-intervention. When overall satisfaction and treatment benefit was assessed, 50% of the spinal cord patients indicated that they had obtained ‘some improvement’ to ‘good improvement’ with neurofeedback. Jensen, et. al. noted that changes were most noteworthy, from pre-intervention to post-intervention, in the Theta band (inhibited) and Alpha band (uptrained), but not in the Beta/High Beta band (inhibited.) Despite its naturalistic design, research-informed protocol inclusion, and counterbalanced protocol presentation, the main study limitations included small sample size and lack of an appropriate control group.
Prinsloo, Novy, Driver, Ramondetta, Eng, Lopez, Lee, Lyle, & Cohen (2017) designed a study that overcame some of design limitations of earlier studies. They studied the effectiveness of neurofeedback in a more prevalent condition involving neuropathic pain, namely Chemotherapy-Induced Peripheral Neuropathy (CIPN). Chemotherapy as a cancer intervention has been associated with at least temporary side effects relating to peripheral neuropathy (e.g. pain, tingling, numbness, burning, etc.) It has been estimated that as many as 91% of chemotherapy clients experience at least temporary CIPN symptoms resulting from exposure to the neurotoxic agents used in chemotherapy. There is no known effective pharmacologic intervention to alleviate these symptoms. Prinsloo et. al. recruited a sufficiently large sample of post-chemotherapy clients (physician-assessed as suffering CIPN) to randomly assign them to a neurofeedback intervention condition (n = 35) versus a no-intervention control condition (n = 36). Among CIPN patients assigned to the neurofeedback intervention, their pre-treatment QEEG patterns (19 channel simultaneous site acquisition, subjected to S-LORETA source density localization analysis), were characterized by cortical over-activation (elevated amplitudes of Beta) in parietal and frontal areas, with abnormally low Alpha amplitude in parietal lobe sites (relative to a non-clinical normative population.)
The neurofeedback protocol involved uptraining of Alpha in the (underactive-within-that-band-range) parietal areas, and inhibiting Beta activity (in the overactive-in-that-band-range parietal and frontal regions). After completing 20 sessions of neurofeedback, 73% of the clients reported significant reductions in pain, numbness, intensity, and unpleasantness of peripheral neuropathic pain, compared to clients in the no-treatment control group (Prinsloo, et. al, 2017). This study has several strengths. Randomized assignment to groups indicates that there were no systematic differences between groups, prior to condition. Use of S-LORETA source localization analysis allowed for identification of common patterns/locations of both underactive and overactive areas, and to renormalize functioning in those areas. Group sample sizes were sufficiently large to allow the use of parametric tests to yield reliable group differences. The only apparent study drawbacks were the lack of a sham neurofeedback control group (to tease out the contribution of expectancy/placebo effects), and follow-up symptom assessments to assess how long the obtained intervention effects lasted.
Relevant Research Studies Involving One-Channel SMR Training at Cz
Shifting to research using one-channel training at Cz to address physiology-related functional problems or symptoms, at least two recent published studies evaluated the role of neurofeedback in addressing fibromyalgia-related pain and related symptoms. Caro & Winter (2011) conducted a non-randomized trial of neurofeedback with 32 fibromyalgia clients. The diagnosis of Fibromyalgia Syndrome was rendered by physicians via clinical and laboratory exams. Outcome measures (administered pre-intervention and post-intervention) included physician-based measures (e.g. ratings of tenderness, global pain, fatigue, psychological distress and morning stiffness.) Conducting 40+ EEG biofeedback (neurofeedback) sessions at Cz, Caro & Winter (2011) uptrained the amplitude of SMR (12–15 Hz), while inhibiting Theta (4-7 Hz) and High Beta (22-30Hz) amplitudes. Almost half (15/32) of the patients completed (pre and post) the Tests of Variables of Attention (TOVA), a computer-administered continuous performance task of sustained visual and auditory attention. Their TOVA data were compared with TOVA data of a fibromyalgia-control sample (no-neurofeedback) sample at similar time intervals. Visual (but not auditory) attention was significantly improved in the neurofeedback subsample, pre to post, relative to the control sample. Physician-rated measures of tenderness, global pain, and fatigue improved significantly from pre-intervention to post-intervention in all neurofeedback clients (with nonsignificant trends for psychological distress and morning stiffness) relative to fibromyalgia controls. The authors concluded the neurofeedback training of fibromyalgia patients appears promising and deserves further study (Caro, et. al., 2011). Inclusion of a non-neurofeedback fibromyalgia control sample, and use of pre-intervention to post-intervention measurement of sustained attention as well as physician-ratings of fibromyalgia symptom intensity were study strengths. Due to the lack of a balanced study design (randomized assignment to conditions), pre-existing differences between the neurofeedback subjects and control subjects cannot be ruled out (as influencing the results of the study).
By contrast, Kayiran, Dursun, Dursun, Ermutlu, & Karamursel (2010) reported a study of neurofeedback with fibromyalgia patients that did include random assignment, and a treatment-as-usual control condition. Fibromyalgia patients receiving care at an outpatient medical clinic were randomly assigned to a Neurofeedback condition (N = 18)) involving delivery 20 neurofeedback sessions (five, 30-minute sessions per week over 4 consecutive weeks). An SSRI control group (N = 18) received Escitalopram 10am qam, for 8 consecutive weeks. Independent raters, blind to group status, administered the Hamilton Depression Scale and Hamilton Anxiety Scale at each assessment period. All participants completed standard self-report symptom measures, such as the Beck Depression Inventory; Beck Anxiety Inventory; Brief Symptom Inventory-Short Form 36; Fibromyalgia Impact Questionnaire; and Visual Analog (facial expression) Scales for pain and fatigue, at each assessment period 6 different symptom assessments in total). At the initial assessment, 9 clients in the neurofeedback group (50%)and 10 clients in the SSRI control group (56%) met diagnostic criteria for Major Depressive Disorder. Symptom assessments (via blind raters, and via patient self-report measures) were conducted at baseline, and repeated at 2, 4, 8, 16, and 24 in the outpatient clinic.
After obtaining resting (pre-intervention) baseline frequency/amplitude measurements at Cz, each the 18 participants in the neurofeedback group was reinforced during each session for increasing SMR amplitudes and decreasing Theta amplitudes (the Theta/SMR ratio having been used as a training index.) The training protocol then shifted to reinforcement of SMR (amplitude increases) alone. In both the neurofeedback and medication control groups, depression scores decreased significantly with both interventions. In the neurofeedback group, the greatest decreases in pain and fatigue scores occurred at week 4, while the greatest decreases in pain and fatigue in the SSRI control group occurred at week 8. Decreases in fibromyalgia-related symptoms/problems and psychological distress symptoms (e.g. depression, anxiety), both rater-assessed and self-report-based, were significantly greater in the neurofeedback group than in the medication control group. The authors concluded that neurofeedback training is an effective intervention for improving the pain, psychological symptoms, and the compromised quality of life associated with fibromyalgia syndrome (Kayiran, et. al., 2010). A non-treatment or sham-treatment control group was not included to control for placebo/expectancy effects. Nevertheless, the study was conducted in an outpatient where, for ethical reasons, all clients were to receive some active form of treatment. The study results may generalize partly to treatment-seeking fibromyalgia outpatients, although the contribution of placebo effects in both conditions (Neurofeedback and SSRI control groups) cannot be teased out because study design limitations.
Longo & Hefland (2016) reported a case study of a 62 year old woman who was status-post breast cancer surgery and chemotherapy. She was administered baseline and post-intervention QEEG measures, and underwent intensive, time-limited neurofeedback (30 total sessions occurring within 2 weeks, consisting of two to three sessions per day). Her presenting symptoms included poor sleep, numbness of the arms and hands, and reduced balance when walking, requiring the use of a cane. Using New Mind training software, three neurofeedback protocols were administered, one of which used an active sensor placement of Cz. Each protocol involved placing an Active (A1) sensor on the scalp, Linked ears referencing (R1, R2), and Ground (G) sensor placement on the scalp at Pz. Amplitude increases in SMR (13-15 Hz) were rewarded at 85%, whereas amplitudes of slow wave activity (2–10 Hz range) were inhibited at 15%. A 10% inhibit was added for activity in the 16-30 Hz range (spanning Beta/High Beta). The client reported significant improvements in cognitive and physical functioning (improvements in all symptoms noted above) from the pre-intervention baseline to post-intervention assessment (Longo and Hefland, 2016.)
The possibility of post-operative cognitive deficit was not (to our knowledge) directly addressed in past neurofeedback studies studying post-surgical clients. The present case study (below) did not specifically address this factor, as well. Nevertheless, the neurofeedback clinician needs to be mindful to the possibility of residual adverse effects of anesthesia, particularly in elderly clients. In the case study presented below, the 58-year-old client spent 9 hours under anesthesia for Orthopedic reconstructive surgery. Two days later, she spent 5 additional hours under anesthesia during surgery to address complications (bleeding in the subdural space just outside the spinal cord). Quite amazingly, the client did not complain specifically of awareness of cognitive difficulties (e.g. foggy brain following anesthesia.) She was focused primarily upon loss of sensation in her legs with inability to walk, and wanted to address those problems. Perouansky & Hemmings, 2009, and Storrs, 2014, review some of the post-surgical complications associated with the use of anesthesia. For example, in many cases there may be a temporary post-surgical period of transient confusion and fatigue that resolves within a few days. However, in some cases of deep anesthesia, subtle cognitive deficits may ensue, and may persist from months to a year or longer (Perouansky & Hemmings, 2009.) Valentin & Carmona (2015) note that cases of ‘Postoperative Cognitive Dysfunction’ occur more often in elderly clients and those of limited education; up to 10% of clients age 65 and older are estimated to have persistent signs/symptoms of POCD three months after the index surgical event (especially after cardiac surgery). On the basis of past effectiveness (in psychiatric and neurologic conditions), Valentin & Carmona (2015) speculate that noninvasive brain stimulation techniques (e.g. Transcranial Direct Current Stimulation), as well as Neurofeedback/ Biofeedback, might be potentially effective future interventions in cases of post-operative cognitive dysfunction.
Study Hypothesis
The present case study explored the effectiveness of SMR training at site Cz in addressing sensory-motor dysfunction, physical pain, and physiological consequences of prolonged stress (exhaustion and resource depletion in the form of entrenched depression/anxiety). The client was a 58 year old married female who presented with residual impairments, including loss of sensation (feeling) in both legs, balance problems, and inability to walk unaided. She had undergone a major surgical removal and reconstruction of her thoracic spinal vertebrae, following by surgical complications. Having undergone 3 months of intensive residential rehabilitation, she had reached maximum benefit from interventions and was discharged (with limited prognosis for further recovery). Based upon a-priori considerations (knowledge of research, brain-behavior correlates, anecdotal reports, and previous experience, etc.), it was hypothesized that a standard course (e.g. 15-20 sessions) of single channel neurofeedback training at Cz – uptraining the amplitude of SMR while placing inhibits on Theta and/or High Beta – would result in gains/improvement in sensation (feeling), sensorimotor functioning, and reduced frequency/intensity of somatically-channeled depression and anxiety.
Method
Subject Characteristics
The participant was previously employed as a television reporter and media consultant, who was well-known in the local area. She had suffered from years of degenerative disk disease, scoliosis, kyphosis, and lordosis, which became increasingly painful and disabling. Degenerative disc disease had caused the ‘shift’ of the vertebrae to rapidly change, prompting a 9-hour surgery involving removal of 14 thoracic vertebrae and their replacement with prosthetic material and support rods. Two days later, on June 10, 2017, the client started bleeding into her spinal column and required an emergency 5-hour surgery to stop the bleeding. The client then spent three months in a spinal cord injury rehabilitation unit of a major U.S. hospital, in an effort to regain the generalized loss of sensation from vertebrae T-8 downward. While the client did learn to move her legs, she was unable to feel them, and hence, unable to walk. The widespread loss of sensation in the lower half of her body was accompanied by difficulty with balance, coordination, and compromise of other sensory-motor functions.
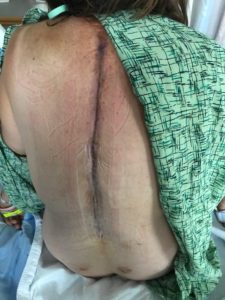
Photo of post-surgical back view provided by client
QEEG Assessment
The client completed a standard neurofeedback assessment, involving acquisition (by the primary author) of a 19 channel QEEG in the eyes closed and open conditions, and completion by the client of the Metabolic Problems Checklist, Cognitive Emotional Checklist, and Interactive Style Inventory (ISI), via the New Mind Expert QEEG System. Those data are displayed in the Results section. Her primary symptoms were poor balance, inability to walk without a walker or cane, and no physical sensation, whatsoever, from her waist down to her feet.
Neurofeedback Training:
Neurofeedback sessions (30 minutes per session) commenced on September 8, 2017, and ended on February 6, 2018 (totaling 15 sessions). New Mind assessment and training software was used at all sessions. While many popular QEEG/neurofeedback software programs do not include the option of one-channel linked-ears montage for training purposes, the New Mind software program does permit this option. Nu-Prep gel was used to clean/prepare each site, and 10-20 paste was applied to attach sensors to the scalp and ears. The montage was configured as follows: the Active sensor was attached to the scalp at Cz (inputted to the A1 slot of the connector cable); Reference sensors were placed on both ears and inputted into two slots of a small plastic ‘jumper’ (containing a small wire connecting the two inputs, thereby linking them); the jumper was then inputted into the R1 and R2 slots of the connector cable; and a Ground (G) sensor was attached to the scalp at site Pz and inputted to slot G of the connector cable. Starting reinforcement rates were as follows: The ‘Reward’ setting was set at 85% (reward for an amplitude increase 85% of the time); an Inhibit setting of 15% for Theta (reward for an amplitude reduction 85% of the time); and Inhibit setting of 10% for High Beta (reward for an amplitude reduction 90% of the time). As such, Sensorimotor Rhythm (SMR; 12-15 Hz) was rewarded at 85%, Theta (4–7 Hz) was inhibited at 15%, and High Beta (21–32 Hz) was inhibited at 10%.
The protocol was modified/customized, based upon the response and comfort level of the client. Some sessions involved eyes closed training, and other sessions used eyes-open training. The intuitive rationale for eyes-open training was to improve balance during walking, an activity during which both of her eyes would presumably be open (she had begun to walk with the aid of a walker). About 2/3 of the way through the sessions, the client reported a recurrence of migraine headaches, a condition she had lived with most of her life. A modification of the 10% inhibit was made, based upon Prinsloo’s research studies, past personal experience (see references by Longo), and anecdotal information from Dr. Richard Soutar, a close associate (who has empirically found that down-training of Beta amplitude at Cz (along with a 10% inhibit on High Beta) is often successful in reducing the intensity and frequency of migraine headaches). The 10% inhibit was reset to the range of 16 – 32 Hz activity (thereby placing a gentle inhibit on the full range of Beta and High Beta activity.) This modification proved effective in ameliorating and then ceasing the client’s complaints of disruptive migraine headache activity. [For additional information on the reduction of migraines, see Walker’s (2011) neurofeedback study, and studies utilizing the HEG-PIR biofeedback method originally developed by Jeff Carmen, Ph.D.]
The client did not complete a weekly symptom tracker. She did, however, provide a session-to-session progress report over the 15 sessions of neurofeedback (which occurred between September 8, 2017 and February 6, 2018), which is presented in the Results section. At the end of neurofeedback session 15, the client, satisfied with her progress and eager to get back to her busy life that included frequent travel, opted to discontinue sessions. She reportedly continues to do well since the sessions ended.
Results
QEEG Assessment Data/Findings
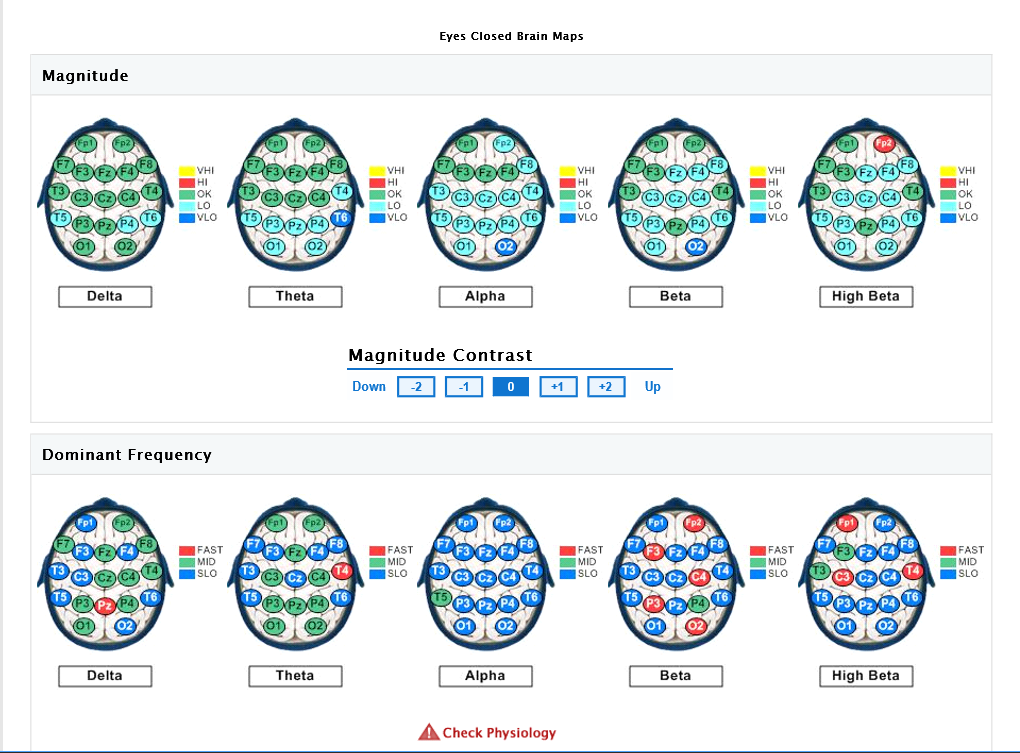
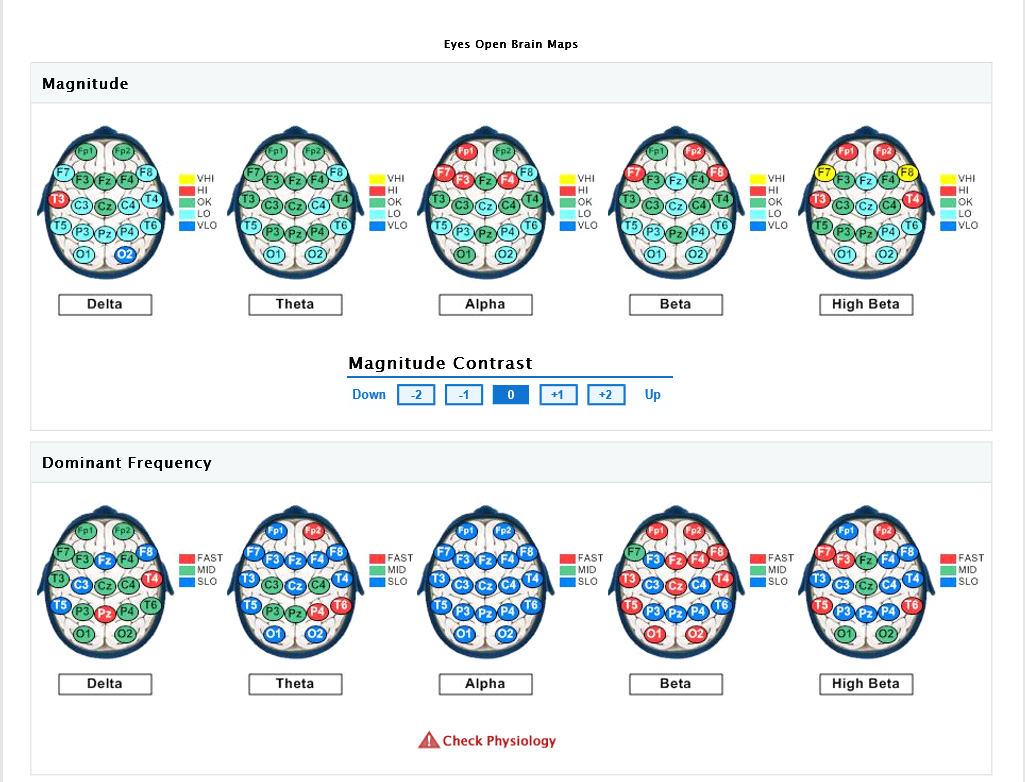
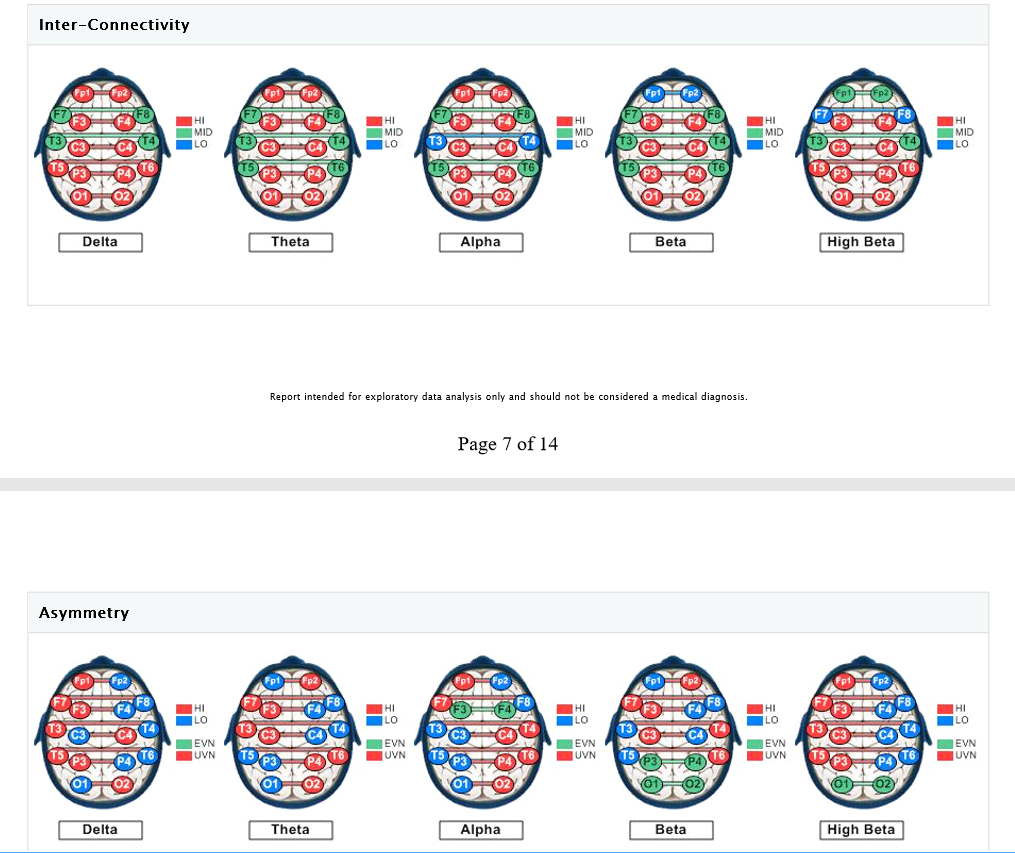
The eyes-closed QEEG data (reflecting resting state or default activity) is remarkable for abnormally low power in posterior Theta, central and posterior Alpha, and central/posterior Beta and High Beta. The Alpha dominant frequency is slowed (lower frequency Alpha), although this could also (due to filter overlap) reflect abnormally high amplitudes in the upper Theta frequency range, which would be consistent with previous research indicating excessive Theta activity in chronic pain (though client was not yet subjectively experiencing chronic pain). Slowed (DF) Alpha suggests reduced cortical and subcortical integration (inefficient resource allocation involving the timing of cortical activation). It may also reflect underlying metabolic or medical factors (which will be reviewed via the Metabolic Checklist results). Hyper-coherence is seen across all frequency bands, suggesting a global lack of processing differentiation. Homologous sites in each hemisphere are over-communicating (to compensate for loss of neural inputs from the spinal cord), resulting in a fragile rigidity/inflexibility – limited degrees of freedom, cognitively, emotionally, and physiologically. This hyper-coherence across frequency bands suggests that the neural reorganization (and compensatory) process that is occurring in both cortical and subcortical regions of the brain is not well-integrated/connected functionally, and hence, inefficient. There may be excessive ‘noise’ in the brain emanating from underactive (high amplitude slow wave activity) and overactive (high amplitude fast wave activity) circuits or networks, compromising the overall stability and efficiency of the brain’s functioning (a low signal to high noise ratio, so to speak.)
Asymmetry analysis indicates the likelihood of excessive Alpha activity (slowing) of the left hemisphere, and excessive fast wave (Beta/High Beta) activity in the right frontal and occipital areas (suggesting underlying anxiety, fear, tension, and vigilance). Concurrent slowed (Alpha elevations) together with Beta elevations of the left hemisphere suggest a locked-in (protective, entrenched) response to prolonged and/or intense stress, possibly characterized by physiological signs of depression (that may be expressed via somatic channels), along with some degree of anxious rumination (even if the client is not fully cognizant of these tendencies, perhaps because she has become accustomed to them.)
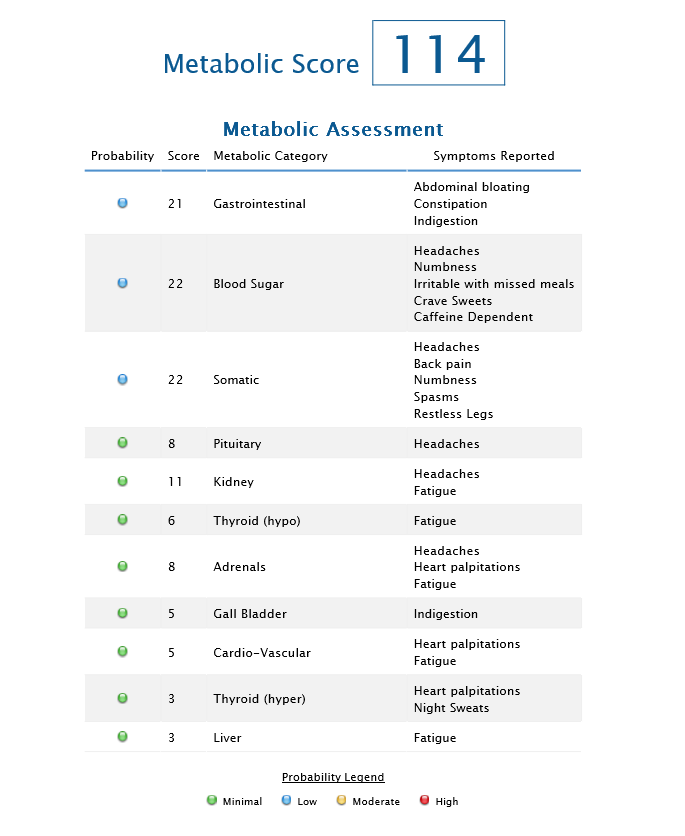
A review of the Metabolic Symptoms Profile indicates that the total score is elevated (consistent with the QEEG pattern of slowed peak-frequency Alpha activity). Symptom clusters that were most elevated suggest gastrointestinal problems, blood sugar instability and somatic difficulties disrupting daily functioning. These metabolic factors, if left unaddressed, could potentially slow the rate/efficiency of progress via neurofeedback. It may be inferred that a great deal the client’s physical, emotional, and cognitive resources are being devoted to ‘defense,’ as she may be struggling to maintain homeostatic balance in her efforts to adjust/compensate for her deficits/irregularities.
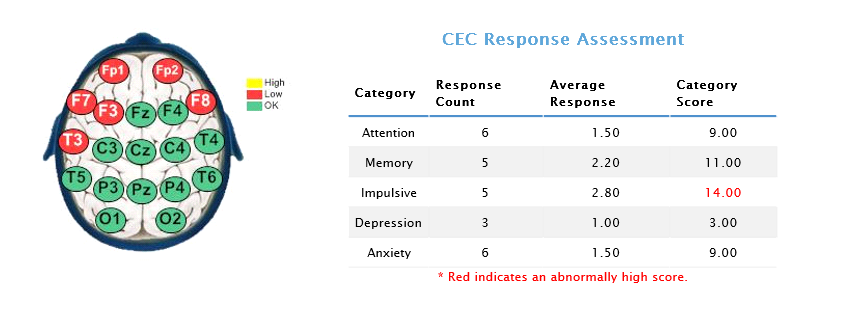
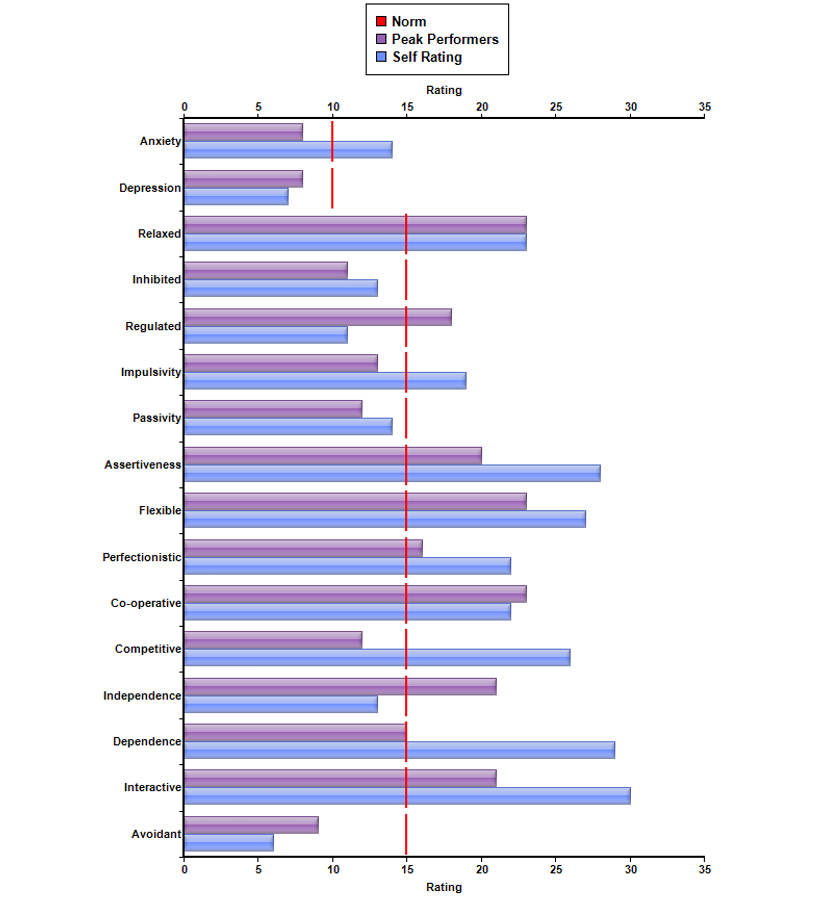
Relatedly, the Cognitive Emotional Checklist (CEC) indicates that she is ascribing to ‘impulsive’ behavior. Presumably, this would refer to emotionally-focused coping strategies, and/or increased emotional reactivity (e.g. underlying grief, anger, anxiety, depression, moodiness, irritability, fear. etc.) Such emotional responses would be a completely normal response to sudden and catastrophic loss – abrupt changes in bodily functioning and the possibility that such changes could remain permanent). The ISI profile suggests that highly emotional behavior is not at all typical for this client, and reflects the immense stress that she has been under, overwhelming her usual coping capacities. For example, her ISI profile suggests that (pre-morbidly), she was a relatively psychologically healthy and resourceful individual, showing many of the characteristics associated with peak performers. Her responses suggest that she usually views herself to be assertive and independent, yet cooperative and interactive. Normally relaxed and well-regulated, she may internally struggle with perfectionistic tendencies (though her highly competitive and visible occupation may demand such behavioral attributes in order to be successful.)
QEEG Brain Map: Eyes Closed
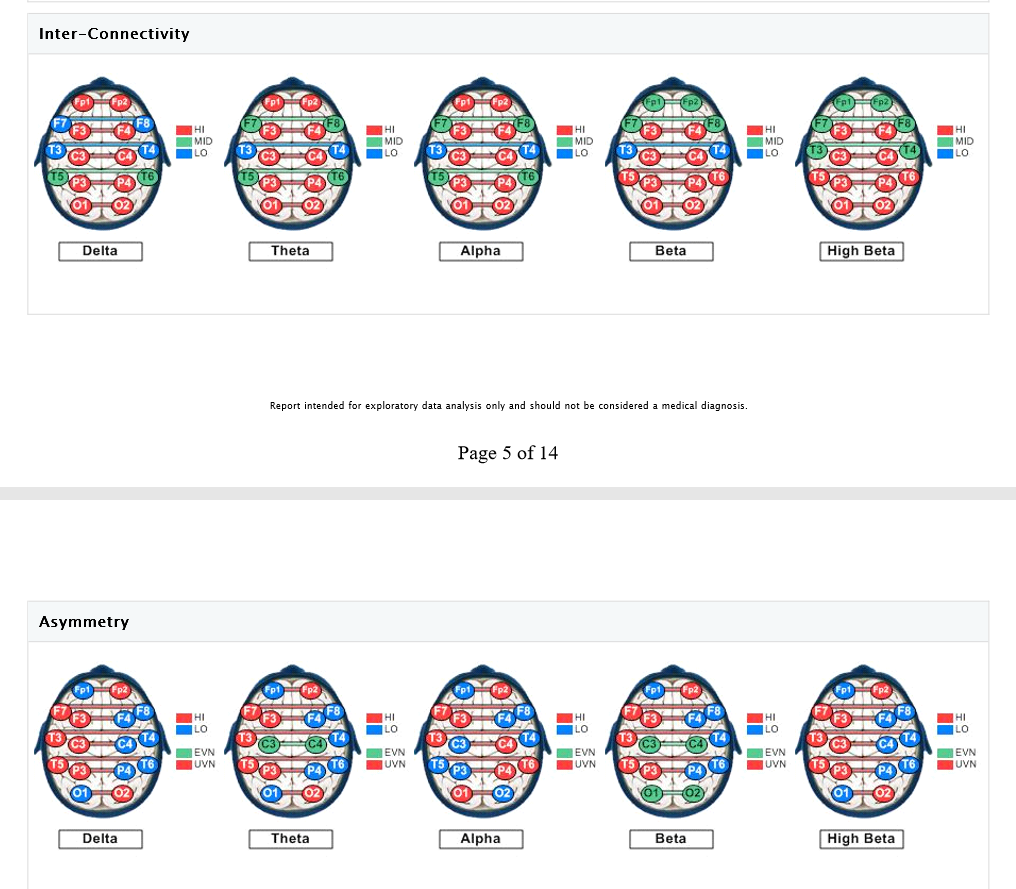
QEEG Brain Map: Eyes Open
Metabolic Checklist Results:
Cognitive Emotional Checklist (CEC):
Interpersonal Style Inventory (ISI):
Through weekly neurofeedback sessions targeting her central nervous system, the client began to report that she was regaining feeling in her legs, and improving her mobility, beginning right after the first session. The client had also reported a lifelong history of proneness to migraine headaches during the assessment process, and these migraines temporarily resurfaced after her sensorimotor functioning (feeling, improved coordination and use of legs) improved.
With the increasing awareness of returning physical feelings/sensations in her legs came a co-awareness of uncomfortable physical pain. The neurofeedback protocol was modified to more broadly down-train the amplitude of fast wave activity (e.g. Inhibit now placed upon 16-32 Hz activity, spanning the full Beta and High Beta ranges) to assist with pain management. High Beta amplitudes have been found to correlate significantly with muscle tension (which can be assessed via EMG.) The client’s reports of pain reduced in intensity with additional sessions. Within one month of starting neurofeedback, the client was able to walk using a cane, and by 7 weeks of training, she was able to walk without a cane. The client reported that lifelong migraine headaches had now ceased by the end of the neurofeedback process. The client chose to discontinue following completion of the 15th session, being a busy person who wanted to get back to her previously busy life, including travel. At the end of training, the client exclaimed, “I have made more gains than any of the medical doctors ever gave me hope for!”
As noted previously, given the clients initial report of symptoms, neurofeedback training began before she had undergone the formal QEEG assessment. Details of the 15 neurofeedback training sessions are presented in the table below. Due to her travel schedule, it was not possible for the client to always have a weekly neurofeedback session. After 15 sessions, she was feeling confident she could continue to maintain and possibly even improve her walking and balance, through additional physical therapy and daily exercise.
| DATE | Service |
| September 16, 2017 | Full 19 Point QEEG E/C E/O |
| September 8, 2017 | NFB#1 – 1 / 1 SMR at Cz E/C 2-7d, 20-30d |
| September 29, 2017 | NFB#1 – 2 / 2 SMR at Cz E/C 2-7d, 16-32d |
| October 6, 2017 | NFB#1 – 3 / 3 |
| October 13, 2017 | NFB#1 – 4 / 4 |
| October 20, 2017 | NFB#1 – 5 / 5 |
| October 27, 2017 | NFB#1 – 6 / 6 |
| November 17, 2017 | NFB#1 – 7 / 7 |
| November 17, 2017 | NFB#1 – 8 / 8 |
| November 22, 2017 | NFB#1 – 9 / 9 SMR at Cz E/C 2-4d, 16-32d |
| December 1, 2017 | NFB#1 – 1 / 10 SMR at Cz E/OPENED 2-4d, 16-32d |
| December 15, 2017 | NFB#1 – 2 / 11 |
| December 21, 2017 | NFB#1 – 3 / 12 |
| January 12, 2018 | NFB#1 – 4 / 13 |
| January 30, 2018 | NFB#1 – 5 / 14 |
| February 6, 2018 | NFB#1 – 6 / 15 |
| February 6, 2018 | DC NFB |
| 15 Total Sessions | |
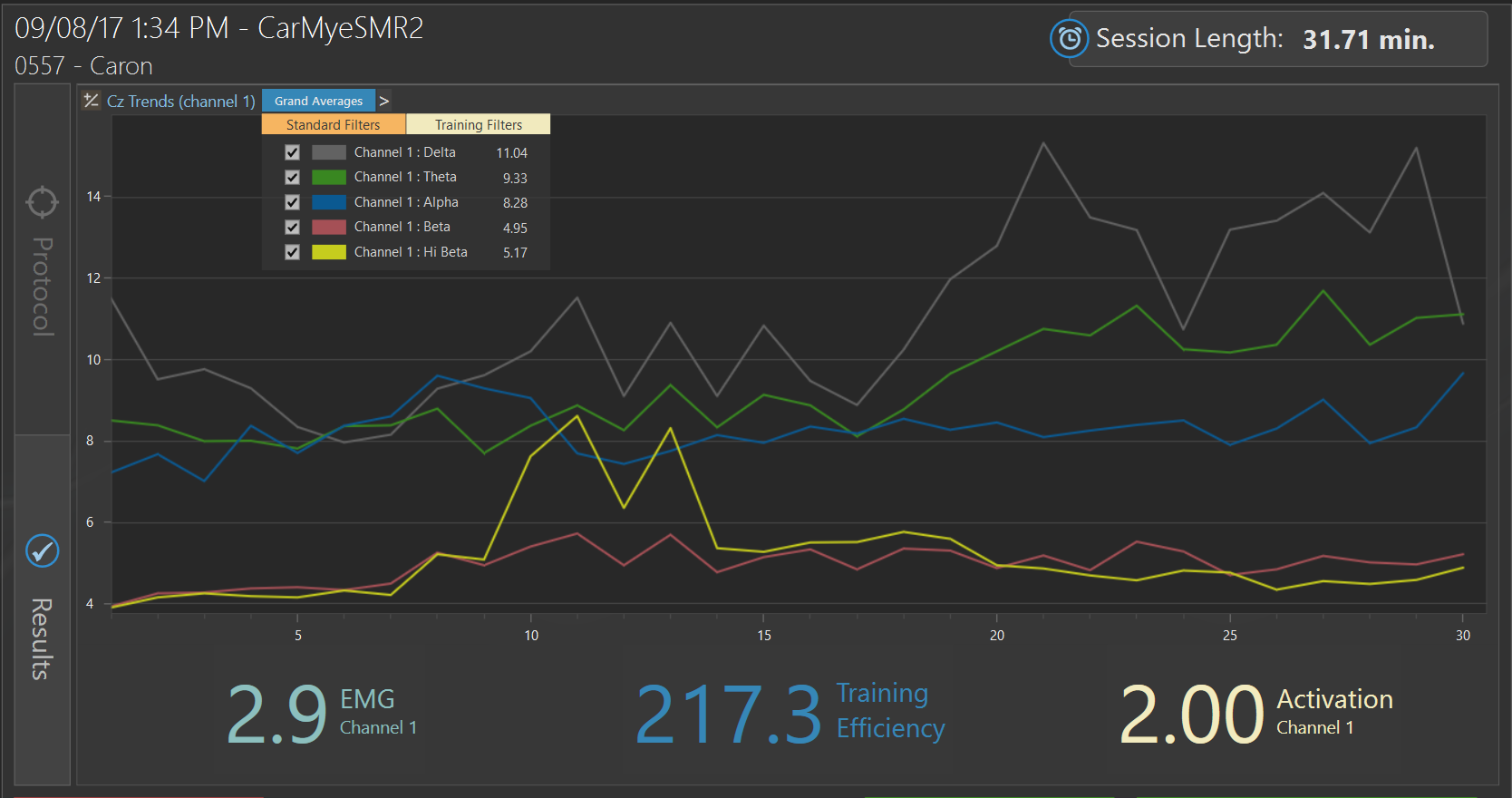
Pre-Neurofeedback Trend Screen Protocol 1
Initial session of neurofeedback at Cz on September 8, 2017
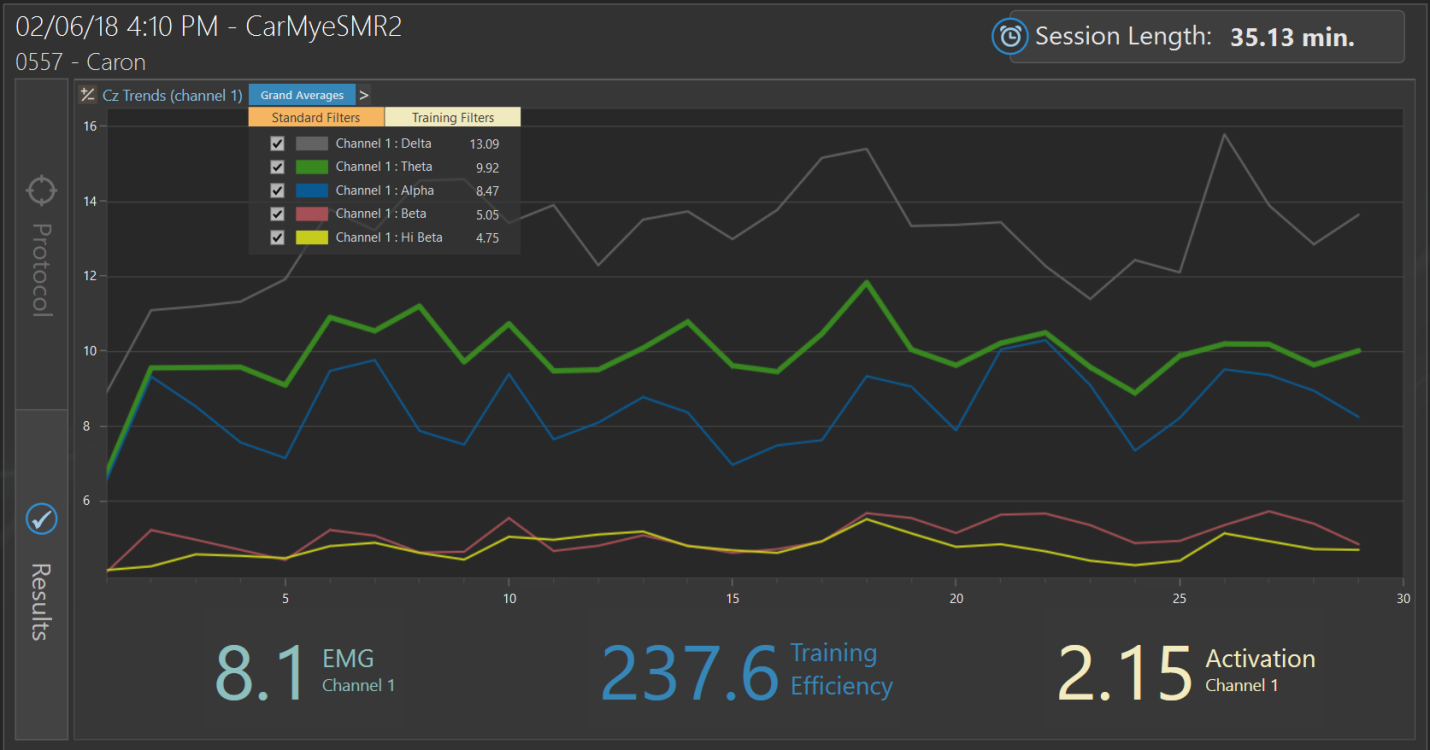
Post-Neurofeedback Trend Screen Protocol 1
Final session of neurofeedback at Cz on February 6, 2018
Weekly Symptom/Progress Report
Excerpts from progress notes in the client’s chart, documenting her self-reported status and week-to-week progress, are presented below:
9/8/17: First session. Client reports recently having undergone spinal surgery resulting in lack of sensation from T8 down, compromised walking and balance and sleep issues. Training session, utilizing SMR uptraining at site Cz. After this session client reported having both feeling and sensation from T-8 through T-11 which was verified by her physical therapist.
9/29/17: Client reports, “How am I feeling? I’ve felt better. Just lots of pain, especially at night. I think the weather is changing.”
10/6/17: Client reports, “Pain was the same, but legs have been in a constant state of “electricity” since then. Like spasms or seizures that I FEEL throughout my body. But only the legs are moving/spasming. I am also aware of feelings in my gut and pain (which I have not felt before). Walking is getting better.”
10/13/17: Client reports, “Increased sensation in feeling in lower back, gut area and left leg. Little to no feeling/sensation in right leg.”
10/20/17: Client reports, “Increased sensation in feeling in lower back, gut area and left leg. Still little to no feeling/sensation in the right leg. Pain is much better. No longer taking pain meds.”
10/27/17: Client reports, “I am walking without a cane and I am feeling sensation in my right foot. Pain is improving.”
11/17/17: Client reports, “I am doing a little better and working on my stamina. Can walk longer without a cane. No improvement with sensation in my right foot/leg. Pain is improving.”
11/22/17: Client reports, “I have more feeling in my right foot and pockets of feelings and sensation in my right leg. I am walking better without a cane.”
12/1/17: Client reports, “Things are just about the same as they were last week. The surgeon has told me I will never work again and things are not going to improve beyond where they are now; and he told me I need to use my cane all of the time. I am able to walk without a cane for up to 4 hours at a time.”
12/15/17: Client reports, “I am walking more and better without a cane. My balance is better, and I have a little more sensation in my right foot toes.”
12/21/17: Client reports, “I am walking more, my balance continues to improve and I have a little more sensation in my right leg.”
1/12/18: Client reports, “Walking and balance are about the same. Headaches have become worse.”
1/30/18: Client reports, “Walking about the same. Balance improving and some more sensation in legs. Headaches have become worse.”
2/6/18: Client reports, “I am doing well.”
Discussion
At the end of treatment, when a client exclaims, “I have made more gains than any of the medical doctors ever gave me hope for!”, it is one of those rewarding moments that the neurofeedback practitioner looks forward to. Successful facilitation of natural healing processes in clients who have suffered injury and loss strengthens the practitioner’s resolve to take on difficult cases – often individuals who have exhausted traditional medical care options.
In this case study, the hypothesis that neurofeedback training at site Cz would facilitate and stimulate further functional gains (in a client with considerable residual sensory-motor impairment) was supported. Remarkably, improvements were reported almost immediately, and these improvements continued (with iterations) until the client was satisfied with her progress – after only 15 neurofeedback sessions.
The client is a high-functioning, highly-motivated individual, who was psychologically healthy and adaptive before the injury occurred (as her ISI profile and occupational attainment would suggest). As she was a resilient and determined individual, neurofeedback served as a catalyst for rapid neural reorganization (neuroplasticity) within the CNS, resulting in at least partial recovery of lost sensorimotor functions. Excessive ‘noise’ within the CNS (e.g. abnormally elevated amplitudes of both slow wave and fast wave activity in the resting state) lessened, permitting a more normal flow of bioelectrical information that facilitated network-to-network communication and efficiency.
The skeptic is likely to question such a remarkable outcome by offering alternative explanations for the findings. Perhaps the medical personnel were premature in their pronouncements that the client had reached maximal functional benefit (recovery) from traditional medical care. Perhaps she would have regained some of these (e.g. sensory, motor, balance) functions over time, anyway. The placebo (expectancy) effect accelerated this process, as the client intensified her efforts (exercise; physical therapy, etc.), reaching gains sooner than she might have otherwise. From a statistical perspective, perhaps she was an ‘outlier’; an extremely unusual and unlikely outcome which simply does not occur among the majority of individuals with similar problems. The skeptic may also muse that, perhaps due to destabilizing effects of intense stress, the client was not able to reliably assess her own states, and capacities. Perhaps she no longer trusted physicians (because of the unexpected complications from surgery that left her in worse condition than before the surgeries), and downgraded the progress that she had made during the rehabilitation stay (the effects of which were continuing at the time she should neurofeedback training). Perhaps she had heard stories of miraculous recovery with neurofeedback, and went into neurofeedback, anticipating such recovery herself.
It is conceded that improvements were based on client self-report, although training screens from the neurofeedback sessions showed a trend in the direction of normalization of brain activity. Certainly, collateral sources of information would have been valuable, such as Orthopedic assessments of range of motion and balance, and EMG nerve conduction studies, pre-surgery, post-rehab., and post-neurofeedback, but such assessments were not feasible and would have been prohibitively expensive.
Study criticisms aside, the present findings are supported by independent research studies that found neurofeedback trials (with slight variations in methodology) to result in findings similar to those of the present study (involving improvements in sensory and/or motor functioning, and pain reduction. Returning to the research initially cited in the literature, it is recalled that Ibric & Dragomirescu (2009) performed neurofeedback trials with a large sample of patients with various neuro-pathologies, reporting that the majority benefited with regard to improved physical function, pain reduction and emotional symptom reduction. Jensen et. al (2007) studied 18 clients with Complex Regional Pain Syndrome, Type I in a multidisciplinary program, noting that all clients reported significantly reduced pain intensity at primary (bodily) pain sites (50% site-specific reduction), with half of the clients reporting at least a 30% overall reduction of pain. In a later study, Jensen et. al. (2013) studied 10 spinal cord patients, and found that 12 sessions of neurofeedback resulted in 70% of patients reporting ‘some’ pre-to-post reduction in ‘worst pain intensity’ and ‘pain unpleasantness’, posttreatment and at a 3-month follow-up. Hassan, et. al. (2016) provided neurofeedback to 7 clients diagnosed with paraplegia (with associated central neuropathic pain of the legs.) Overall, 4/5 clients reported a clinically significant reduction of pain (>30 % pain reduction) lasting at least a month beyond the intervention. Prinsloo, et. al. (2017) reported in a randomized study of post-chemotherapy neuropathic pain patient that the neurofeedback group reported greater reductions in the intensity of neuropathic symptoms than did non-treatment controls. Caro & Winter (2011) and Kayiran, et. al. (2010) found neurofeedback to significantly reduce fibromyalgia symptoms, with latter study showing neurofeedback to be superior to 8 weeks of SSRI medication (treatment-as-usual control group. In a case study using methodology similar to the present study, Longo and Hefland (2016) reported that brief, intensive neurofeedback resulted in improvements in sleep, reduced numbness of the arms and hands, and improved balance when walking, in a post-chemotherapy patient. Taken as a whole, these studies demonstrate an empirical precedent for the use of neurofeedback in facilitating improvement of symptoms associated with a range of neuropathologic conditions, including spinal cord injury/paraplegia, Regional Complex Pain Syndrome, neuropathic pain, and fibromyalgia-related symptoms. Neurofeedback-facilitated functional and symptomatic improvement extends to problems including sensory loss, motor and balance problems, chronic pain, and emotional distress.
The study further supports the proposal that one and two channel amplitude training continues to contribute to impressive clinical outcomes. The observation that variations in protocol can be used, and yet similar outcomes be obtained, supports the characterization of Neurofeedback as a robust and flexible methodology. Clinical experience, acumen, and creativity will always remain invaluable, as neurofeedback is so much more than a plug-and-play technology. Experienced practitioners who have profited from trial-and-error experience and who are highly attuned to subtle behavioral changes, can make protocol adjustments and tweak reinforcement rates on-the-fly, to optimize a client’s training response and thereby achieve impressive results quite efficiently.
Case study designs, while naturalistic and ecologically valid, have their limitations. Because of minimal control of factors and events that operate outside of the neurofeedback session (e.g. nutritional and metabolic factors; lifestyle issues; genetics; concurrent medical or holistic interventions; individual psychotherapy, marital or family therapy; effects of prescription and over-the-counter medications; use of supplements; use of illicit substances; exposure to toxins and/or environmental pollutants; degree of family support, life stress, random and chance factors, etc.), direction of causality cannot be established. One cannot assert, with confidence, that neurofeedback training alone ‘caused’ the outcome reported by the client. There may be other factors influence the outcome, or synergistic effects of various factors.
Case studies are promising ways of identifying approaches and methods that appear worthy of further study, using more sophisticated study designs. Causal directionality statements require tight experimental control over many variables, together with representative (unbiased) sampling and randomization. Placebo control conditions are typically included in studies of active interventions, to control for expectancy effects (which are, in and of themselves, powerful). Sham neurofeedback is sometimes incorporated as a control condition in contemporary neurofeedback studies. Siegfried Othmer, a leading neurofeedback authority, has argued that sham neurofeedback (as it is usually practiced) is often ineffective as a control condition, because the subject quickly senses a disconnect (incongruity) between the patterns of information shown on the screen and their internal subjective experience. Creative design changes to make sham interventions as realistic as possible are clearly needed. At present, there is no known artificial intelligence that can duplicate the rapid and creative reasoning and decision-making processes that characterize the work of the talented neurofeedback clinician – processes which become more implicit and intuitive with time and experience.
Statistically, there is always the possibility that single study findings are chance occurrences, particularly when the study utilizes a limited sample size (potentially violating assumptions of parametric statistical tests typically used to identify statistically significant differences on interval-scale measures). There is also the issue of sampling/selection bias. With government and private funding of independent neurofeedback research almost non-existent these days, practicing clinicians have had, out of necessity, to limit their scope of inquiry to those clients who come to them (or their agencies) seeking intervention. It is hoped that as neurofeedback research findings continue to accrue (with refinements in research technique and design, and application to various problems and populations) more and more healthcare consumers will come to seek neurofeedback as a first or second intervention choice (rather than as a last resort). When that happens, greater interest and scrutiny will be placed upon neurofeedback, and funding opportunities for research (including controlled laboratory studies) will open up.
From the current study, viewed in the context of previous research, it may be concluded that neurofeedback, at its most basic level, acts as a catalyst to stimulate functional improvements and reduction of dysfunctional symptoms (by hypothetically facilitating the process of neural reorganization in the direction of positive neuroplasticity.) This is not merely conjecture, as two previous studies employing fMRI technology found significant structural changes (volumetric) in gray and white matter after a typical trial (typically lasting 20 to 40 sessions) of neurofeedback (e.g. Levesque, Beauregard, and Mensour, 2006; Gharizi et. al., 2013.) Each case study and small N study that is reported adds to our confidence that effective and affordable help is available to clients, especially to those deemed by traditional medicine to have limited likelihood of future improvement.
References
Caro, X.J., & Winter, E.F. (2011). EEG Biofeedback Treatment improves certain attention and somatic symptoms in Fibromyalgia: A pilot study. Applied Psychophysiology and Biofeedback, 40, 251-256.
Gharizi, J., Tucholka, A., Larue, V., Blanchette-Sylvestre, M., Reyburn, G., Gilbert, G., Levesque, J., and Beauregard, M. (2013). Neurofeedback training induces changes in white and gray matter. Clinical EEG and Neuroscience, 44, 265-272.
Hassan, M. A., Fraser, M., Conway, B.A., Allan, D. B., and Vuckovi, A. (2015). The mechanism of neurofeedback training for treatment of central neuropathic pain in paraplegia: a pilot study. BMC Neurology, 15. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-015-0445-7
Hopson, J., Soutar, R., and Longo, R. (2017). Correlating Oxidative Stress and QEEG, New Mind Online Journal, www.nmindjournal.com
Ibric, V. L., & Dragomirescu, L. G. (2009). Neurofeedback in Pain Management. In T. M. Budzynski, H. K. Budzynski, J.R. Evans, & A. Abarbanel (Eds.), Introduction to Quantitative EEG and Neurofeedback: Advanced Theory and Applications, 2nd Edition, 417-451. Boston, MA: Academic Press.
Jensen, M. P., Grierson, C., Tracy-Smith, V., Bacigalupi, S. C., and Othmer, S. (2007). Neurofeedback Treatment for Pain Associated with Complex Regional Pain Syndrome Type I, Journal of Neurotherapy, 11, 45-53
Jensen, M.P., Gertz, K.J., Kupper, A.E., Braden, A.L., Howe, J.D., Hakimian, S, & Sherlin, L.H. (2013). Steps toward developing an EEG Biofeedback treatment for chronic pain. Applied Psychophysiology and Biofeedback, 38, 101-108.
Kayiran, S., Dursun, E., Dursun, N., Ermutlu, N., and Karamursel, S (2010). Neurofeedback intervention in Fibromyalgia Syndrome: A randomized, controlled, rater-blind clinical trial. Applied Psychophysiological Biofeedback, 35, 293-302.
Levesque, J., Beauregard, M., and Mensour, B. (2006). Effect of neurofeedback training on the neural substrates of selective attention in children with attention deficit/hyperactivity disorder: A functional magnetic resonance imaging study. Neuroscience Letters, 394, 216-221.
Longo, R.E. (2018). A consumer’s guide to understanding QEEG brain mapping and neurofeedback training. iUniverse: Bloomington, IN.
Longo, R. E., & Helfand, D. (2016). Treating Post-Chemotherapy Symptoms with Neurofeedback. NeuroRegulation, 3, 92–97. http://dx.doi.org/10.15540/nr.3.2.92
Longo, R.E. (2015). The use of neurofeedback to treat traumatic brain injury in sexually abusive youth. In, Prescott & Wilson (Eds.), Very different voices: Perspectives and case studies in treating sexual aggression. Holyoke, MA: NEARI Press.
Montgomery, D., Robb, J., Dwyer K. V., & Gontkovsky, S. T. (1998). Single channel QEEG amplitudes in a bright, normal young adult sample. Journal of Neurotherapy, 2, 1-7.
Nunez, P.L . & Srinivasan, R. (2006). Electric Fields of the Brain: The Neurophysics of EEG, 2nd Edition. New York, NY: Oxford University Press.
Othmer, S. (2016). History of Neurofeedback. In H.W. Kirk (Ed.), Restoring the brain: Neurofeedback as an integrative approach to health. Boca Raton, FL: Taylor & Francis Group.
Othmer, S., & Othmer, S., Infra-Low Frequency Training. eeginfo.com/research/infra-lowneurofeedback.jsp
Perouansky, M., & Hemmings, H. C. (2009). Neurotoxicity of general anesthetics: Cause for concern? Anesthesiology, 111, 1365–1371.
Prinsloo, S., Novy, D., Driver, L., Ramondetta, C., Eng, G., Lopez, R., Lee, R., Lyle, R., & Cohen, L. (2017). Randomized controlled trial of neurofeedback on Chemotherapy-Induced Peripheral Neuropathy: A Pilot Study, Cancer, 123, 1989-1997.
Sime, A. (2004). Case study of Trigeminal Neuralgia using neurofeedback and peripheral biofeedback. Journal of Neurotherapy, 8, 59-71.
Soutar, R., and Longo, R. (2011). Doing Neurofeedback. McLean, VA: ISNR Research Foundation.
Storrs, C. (2014). The hidden dangers of going under. Scientific American. Retrieved from http://www.scientificamerican.com /article/hidden-dangers-of- goingunder/?&WT.mc_id=SA_MB_20140319
Valentin, L.S.S., & Carmona, M. J. C. (2015). A review of postoperative cognitive dysfunction: Diagnostic and rehabilitation. Principles and Practice of Clinical Research, 1, 28-33.
Walker, Jonathan (2011). QEEG -Guided Neurofeedback for Recurrent Migraine Headaches, Clinical EEG and Neuroscience, 42, 59-61.