Clinician Name: Chris Branciere MRC, BCB
Clinic Name & Location: Biofeedback Associates of Northeast Florida
Professional Status: Chris Branciere, MS is a board-certified biofeedback therapist, part-time psychology professor, and PhD student
Abstract
This is a pilot case-study in which the subject was exposed to dual frequency photic stimulation using pulsating monochromatic yellow light ranging between 13.5-18 Hz while eyes were closed for 16 sessions. Rhythmic flickering lights are presented to stimulate brainwave entrainment and often result in loosening of orientation to external reality and shifts to internal focus, which are products of therapeutic dissociation. Color-coded topographic brain maps or quantitative electroencephalography (QEEG) were recorded during eyes open and eyes closed conditions prior to the intervention and the QEEG was interpreted as reflecting globally low magnitude baseline brainwave activity, particularly in the alpha and beta bandwidths. The baseline QEEG recordings were compared to the brainwave frequency distributions of age- and gender-matched averages to target the most statistically deviant brainwave activity. Normalization of the EEG distribution was apparent in the first post-test EEG recording and maintained following a second post-test EEG recording.
Keywords: brainwave entrainment, photic stimulation, visual entrainment, dissociation
Introduction
Photic stimulation (PS) is a neuromodulatory intervention in which brainwave entrainment occurs through visual stimulation (Frederick, et al., 1999). In this process, brainwave frequencies align with frequencies of observable light stimuli. PS induces hypnotic trance effects, facilitates reframing feelings, modulates pain, and promotes self-regulation, functional connectivity, and integration, in which participants turn inward from external stimuli (Bowins, 2012; Budzynski, Budzynski, Evans, & Abarbanel, 2009, p. 209; Fisher, 2001; Kroger, Schneider, 1959; Siever, 2002; Tang, Riegel, McCurry, & Vitiello, 2016). Brainwave frequency patterns are sustained in the process, in which participants clinically dissociate through either closed loop or open loop systems. Closed loop photic stimulation is contingent upon monitoring EEG activity with an external device, in which brainwave activity is continually measured and fed back to participants through modified light frequency stimuli, which can be used to shape neural output – that is, subsequent brainwave activity is potentially based on the presentations of varying light frequencies that stimulate or entrain brainwaves. Open loop photic stimulation differs in that it is a feedforward system (independent of EEG measurements) in which light stimuli are presented in a predetermined pattern conducive to entrainment.
Trance-like states of dissociation have traditionally been considered pathological. But during PS, subjects are presented with the opportunity to mentally process tolerable levels of engagement and detachment to securely develop and reconfigure neural connectivities in intersubjective space, which is central to the hypnotherapeutic effects of dissociation (Diamond, 2020; Peebles, 2018).
The purpose of this case study is to administer CPS using yellow light pulses at 13.5-18 Hz and to examine potential changes in brainwave activity and subjective states in a healthy volunteer presenting with low alpha and beta production. Brainwave entrainment using color photic stimulation is a safe, non-pharmaceutical method of inducing therapeutic dissociation, in which participants undergo memory enhancement, integrative experiences, internal orientation, and embodiment.
Literature Review
PS is known to elicit the brain to reach a state of entrainment as a therapeutic dissociative state that arises in 80% of participants within the first six minutes of exposure (Kroger & Schneider; 1959, p. 93; Siever, 2002). Sappey-Marinier, et al. (1992) repeatedly recorded 250% increased lactate levels (a correlate for glucose metabolism and neuronal activity) in the visual cortex within 6.4 minutes of initiating PS (p. 587). Declining lactate production was reported with continuous PS, consistent with therapeutic goals of increasing arousal thresholds and reduced neuronal reactivity as adaptive responses. Fox and Raichle (1985) reported 28% increased regional cerebral blood flow (rCBF) in the striate cortex while PS training. In addition to improving memory deficits and other pathological dissociative symptoms, brainwave entrainment has been demonstrated to effectively modulate pain, circadian rhythm shifts, behavioral disorders, and PTSD (Alkozei, et al., 2016; Budzynski, Budzynski, Evans, & Abarbanel, 2009, p. 209; Budzynski, Jordy, Budzynski, Tang & Claypoole, 1999; Carter & Russell, 1993; Figueiro, Bierman & Rea, 2013; Joyce & Siever, 2000; Kroger, Schneider, 1959; Siever, 2002, p. 15; Roberts, Clarke, Addante, & Ranganath, 2018; Tang, Riegel, McCurry, & Vitiello, 2016, p. 39; Tamarova, Limansky & Gulyar, 2009; Tang, Vitiello, Perlis, & Riegel, 2015; Zhang, et al., 2015). Light exposure has also been shown to influence the brain through nonvisual pathways that support cognitive neural networks (Alkozei, et al., 2016; Bajaj, et al., 2017).
Mechanisms of PS vary and even though it was traditionally believed visual entrainment occurred simultaneously through steady-state responses, driving fundamental frequencies (first harmonic) and many individuals have been reported to maximally respond in that fashion, neurons also synchronize more indirectly or (if non-sinusoidal waves are presented) through stimulating different harmonic reactions other than the stimulating frequencies (Collura & Siever, 2008; Heinrichs-Graham & Wilson, 2012; Lazarev, Infantosi, Valencio-de-Campos, & de Azevedo, 2004; Lazarev, Simpson, Schubsky, & Deazevedo, 2001; Roberts & Robinson, 2012). PS more directly enhances cortico-thalamic circuits in the frontal, posterior regions. The temporal lobes are indirectly enhanced. Presentation of pulsed lights also activates the locus coeruleus and other brain structures, stimulating cerebral hormonal changes, blood flow, myelinated nerve recovery, and cognitive functions (Alkozei, et al., 2016; Bajaj, et al., 2017; Dijk & Archer, 2009; Meltzer, et al., 1998; Phipps-Nelson, Redman, Schlangen & Rajaratnam, 2009). Signals are partly mediated by retinal melanopsin cells (Berg & Siever, 2009). Dual frequency entrainment of brainwaves may be facilitated by contralateral stimulation – simultaneously presenting faster pulsing lights to the right field of vision (mostly correlating with the left-brain hemisphere) relative to the left field (correlating to the contralateral hemisphere), thus coinciding with neurotypical asymmetric hemisphere specialization (Auerbach, Beller, Henkes, & Goldhaber, 1961; Rutter, 1987).
Research on clear glasses using polychromatic white lights has amassed for over 80 years (Adrian & Matthews, 1934). PS recently became increasingly popular with more affordable and portable devices. Color photic stimulation (CPS), matching hue ranges with corresponding light frequency pulses in accordance with Lüscher’s (1971) research on the effects of color, may prove to be more effective than monochromatic white light (Ali, 1972; Alkozei, et al., 2016; Bajaj, et al., 2017; Carruthers, Morris, Tarrier, & Whorwell, 2010; Cohen & Hunter, 1978; Cowan, et al., 2008; Dijk & Archer, 2009; Figueiro, Bierman & Rea, 2013; Geerdink, Walbeek, Beersma, Hommes, & Gordijn, 2016, para. 1; Hiroshi, 1987; Lack & Wreight, 1993; Meltzer, et al., 1998; Münch, 2014; Nolan, Dai, & Stanley, 1995; Phipps-Nelson, Redman, Schlangen & Rajaratnam, 2009; Rahimi, et al., 2013; Ruiman, Jingli & Zhen, 1997; Siever, 2014; Siever, 2016; Tamarova, Limansky, & Gulyar, 2009; Tarrier & Whorwell, 2010; Yatsenko, Kaigorodova, Prokhorov & Rastyagaeva, 2015). The presentation of each color at different frequencies stimulates varied and complex brainwave frequencies (Hiroshi, 1987). CPS promotes recovery and performance as the brain is trained to operate more efficiently through the thalamus, facilitating sensory input to the amygdala and hippocampus as well as the corresponding feedback from other subcortical structures. Mood and personality features influence color preferences and reactions. Longer to shorter wavelength hues are matched to potentially amplify the effects of CPS with fast to slow brainwave frequencies corresponding with more to less neural activation. Red light is generally most stimulating and increases high beta, yellow increases low beta, green increases alpha, blue increases slower alpha and these goals may be addressed by offering a combination of dual frequency stimulation with different combinations of color presented separately to each visual field (Ali, 1972; Hiroshi, 1987; Lüscher, 1971; Münch, 2014; Siever, 2014; Siever, 2016). Exposure to red light at 15 Hz using square waves may elicit photo-convulsive episodes in susceptible individuals, though (Fisher, et al., 2005; Siever, 2014; Takahashi & Tsukahara, 1976). Enhancing alpha and beta activity has also been associated with wakefulness, consciousness, focus, and enhanced memory encoding (Bonnefond & Jensen, 2012; Jensen & Mazaheri, 2010; Meeuwissen, Takashima, Fernández, & Jensen, 2011; Roohi-Azizi, Azimi, Heysieattalab, & Aamidfar, 2017).
Dissociation is a neurocognitive trait or reaction to trauma often marked by dysfunctional attentional networks that may be pathological or non-pathological with a variety of symptoms, ranging from memory deficits; impaired integration of thoughts and emotions; increased fantasy proneness, pseudomemories, and disembodiment (Giesbrecht, Jongen, Smulders, & Merckelbach, 2006; Merckelbach, Miskovic & Keil, 2015; Muris, Horselenberg, & Stougie, 2000; Rabellino, et al., 2018). Although pathological dissociation is a core feature of trauma, borderline personality disorder, eating disorders, body shape dissatisfaction, drug abuse, threats to identity, reductions in awareness, memory, cortical interconnectivity, disinhibitory behavior, and hypoemotionality, purposeful or directed dissociative states on the non-pathological end of the dissociation spectrum have been induced using PS, in which subjects re-stabilize, increase parasympathetic activity, connectedness, and integrate memories as adaptive processes of clinical goals (Beato, Rodríguez Cano, & Belmonte, 2003; Bob, Golla, Epstein, & Konopka, 2011; Dalton & Huang, 2013; Dickie, Brunet, Akerib, & Armony, 2011; Edge, 2001; Edge, 2004; Fox & Raichle, 1985; Kunst & Hohle, 2016; La Mela, et al., 2010; Leonard, Telch, & Harrington, 1999; McShane & Zirkel, 2008; Mentis, et al., 1997; Rothgerber, 2019; Sierra & Berrios, 1998; Soffer‐Dudek, Todder, Shelef, Deutsch & Gordon, 2019; Siever, 2015; Soffer‐Dudek, et al., 2019; Spitzer, Barnow, Freyeberger, & Grabe, 2006; Waller, et al., 2001). Therapeutic dissociative induction arises from the presentation of repeated external stimuli, facilitating adaptive mental detachment, displacement, and absorption, creating distance from anxiety before increasingly activating the prefrontal cortex with less limbic activation, which promotes stimulus discrimination and integrative brain activity (Fisher, 2014; Meyerson & Konichezky, 2011; Vazquez, 2005).
There are different reports in the literature about which neural correlates are most closely associated with dissociative states or traits. Decreased occipital and frontal alpha and excess right frontal beta frequencies are commonly recognized EEG markers of trauma and dissociation (Jokić-Begić, & Begić, 2003). Dissociative-type symptoms of reduced neural processing, narrowed attention, and withdrawal, are sometimes reflected in EEG imagery as reduced interconnectivity or hypocoherence (Soffer‐Dudek, Todder, Shelef, Deutsch & Gordon, 2019). Russ, et al (1999) also found that total theta power (based on 16 recording channels) was correlated with dissociative experiences (reduced pain perception) in response to noxious stimuli. Dissociative-type symptoms of reduced neural processing, narrowed attention, and withdrawal are reflected in EEG imagery as reduced interconnectivity or hypocoherence (Soffer‐Dudek, Todder, Shelef, Deutsch & Gordon, 2019). But at least in the temporal lobe, Krüger, Bartel, and Fletcher (2013) found relative decreases in theta with increased alpha ratios, which may relate to reduced memory encoding and recall. Generally, increased alpha power is associated with more positive dissociative states (Lynch & Paskewitz, 1979).
Subject Background
The subject for this case study was a healthy 36-year old middle-class, right-handed married female, who grew up attending public school and completed some college. She had no history of head trauma but was sexually molested as a small child and parents separated when she was 10 years of age. She began dating at 14 years of age. Currently, she has one close friend and is not involved in social groups. She has no suicidal thoughts. She has participated in yoga but no longer exercises. Although there was alcohol dependency on her mother’s side of the family, she engages in no substance abuse. The presenting symptoms were fatigue, apathy, low thermoregulation, ringing in ears, dizziness, mildly self-deprecating, non-restorative sleep, and experiences of mild but frequent headaches. Based on the first QEEG summary report, the subject’s mood was mildly depressed, likely irritable with missed meals, often lethargic, inhibited, and she experiences memory problems. Metabolic issues included moderate digestive problems and a few food intolerances.
An A-B-A-B design was conducted, and EEG was derived by applying 19 cephalic electrodes (constructed of sliver/silver-chloride) based on the International 10/20 system using Brainmaster Discovery 24e hardware – 19 channels. The participant gave informed consent for research purposes and subscribed to topographic mapping of QEEG measured at baseline. These initial brain maps were considered in establishing the protocol (to target statistically deviant brainwave activity) of presenting moderately fast yellow lights as the potentially beneficial intervention for entraining corresponding brainwave frequencies. CPS using yellow light may help attenuate depression. The decision to present yellow lights in this study was partially based on stimulatory effects of viewing yellow on arousal and mood (Carruthers, Morris, Tarrier, & Whorwell, 2010; Cohen & Hunter, 1978; Lüscher, 1971; Nolan, Dai, & Stanley, 1995). The subject was exposed to contralateral photic stimulation, in which lights flickering at moderate rates were presented to the left visual field, stimulating brainwave frequencies in the right hemisphere of the brain (where they are initially registered), while faster pulsing lights were presented to the right field of vision, stimulating the left brain hemisphere differentially. The asymmetrical photic stimulation coincides with neurotypical patterns of brain activity. The training consisted of 8-sessions (24 minutes each) of a spaced practice schedule using the David Delight Pro visual entrainment and Tru-Vu Omniscreen Multi-Color Eyeset. This device produces a sinusoidal wave that prevents harmonic effects. A second set of QEEG brain maps was completed for comparison to the baseline eyes open and eyes closed conditions using the NewMind Clinical Database Report System. After the post-test results were obtained, a two-week washout-out period (in which the intervention was withdrawn), ensued before the intervention was reinstated with an additional eight sessions of PS. A third set of brain maps was completed to compare the neurometric recordings of the second post-test results with the baseline and first post-test brain maps. The qEEG recordings were also compared to an age- and gender-matched normative database.
The 16-session training program was designed to stimulate the sensory motor rhythm (through illuminating the left visual fields of both eyes) in the right brain hemisphere and faster beta brainwave frequencies (through the right visual fields) in the left hemisphere by dispersing flickering yellow lights from LEDs behind a translucent screen on the eyeset, while eyes were closed. The light intensity levels were set at approximately 800 lux, spread across two square inches over each eye.
Results:
In contrast to the low-powered baseline recordings, pronounced normalization of the EEG distribution appeared across brain regions between pre and post-intervention maps, particularly with eyes closed, in terms of global beta, global theta, and frontal, parietal, and posterior alpha magnitudes. The pre/post intervention midline analyses for conditions clearly indicate neuroenhancements across bandwidths and cortical areas, even with increased magnitude in the temporal lobes. The first post-intervention brain map showed the most responsivity in the beta band with the alpha band also largely responsive. The second post-intervention showed the most responsivity in the alpha band with the beta band largely responsive, too. Other EEG bands were normalized to lesser degrees.
During the first two PS sessions, the participant conveyed that she felt relaxed and pleasantly surprised that she briefly fell asleep. Outside of the PS sessions, the participant recalled frequent intentions to take naps but then noticed although she was relatively relaxed, she really did not feel as though she needed to nap. By the third session, a self-affirmation emerged in which the participant noticed she felt more capable, repeatedly telling herself, “I can do this.” The participant also found herself “wondering if this is how Ironman feels.” This seemed to confirm a stronger sense of embodiment she experienced, and this is typical when shedding dissociation. She reported feeling more persistence even during routine tasks, experiencing more focus, yet more relaxation, too. Additionally, she experienced less fatigue. Even though the participant expressed that she was more productive and seemed increasingly assertive and punctual, she was self-conscious about occasional bouts of irritability. But the participant’s daughter reported that her mom was “calmer, not as uptight, and not as much OCD-like.” At this stage, the participant was apparently able to “roll with things without getting upset.” Such interruptions of negative reinforcing behaviors and evolving flexibility occur during photic entrainment and therapeutic dissociation. After the 5th session, the participant postponed the following session five additional days and her daughter spontaneously asked her mom when she was doing her next PS session, stating, “you need it.” The most profound differences in EEG recordings were evidenced in the elevated posterior alpha and beta band activity, which were observed by the eighth session. These changes also coincided with the subject’s increased attention, processing speed, and reduced confusion. The adaptive changes the subject experienced within the first eight weeks were maintained during the eight weeks that followed the first post-intervention brain map. During weeks 9-16, the participant often reported that she felt no further differences or about the same as the first eight weeks. The subject’s microsleeps during PS were increasingly common and sleep improved overall at night, too. She stated she looked forward to the PS sessions and found them to be comfortable and relaxing, despite the intensity of the flickering lights. Even on days following PS exposure, the subject was surprised that she didn’t feel as though she needed naps to restore herself. Three weeks following cessation of PS training, the participant conveyed that she was not sleeping as well and that she found it a little more difficult to get out of bed. But she also realized that she no longer had a phobia of vomiting. She expressed an intention to purchase a photic stimulation device.
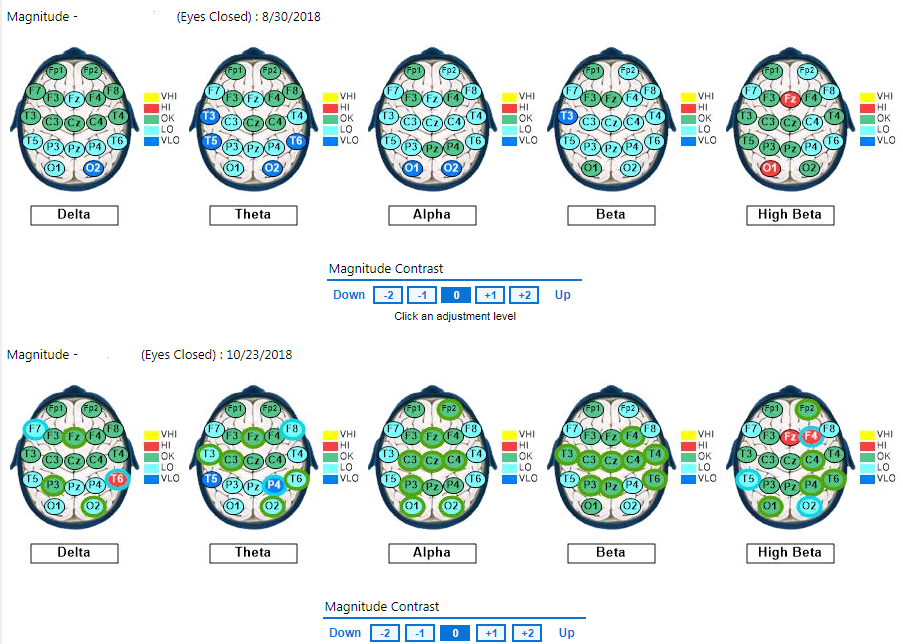
Figure 1. Baseline eyes open qEEG compared to qEEG following eight PS sessions (2nd Brainmap)
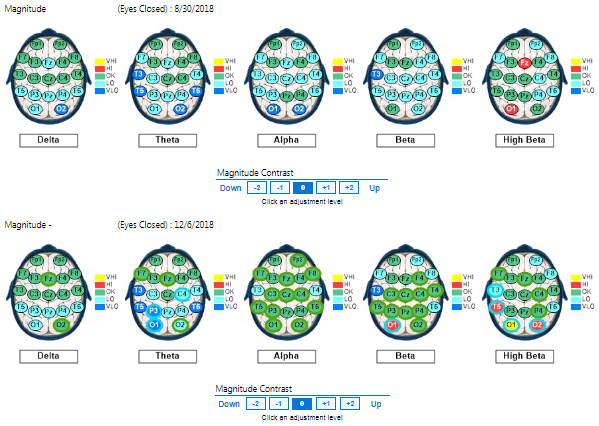
Figure 2. Baseline eyes open qEEG compared to qEEG following 16 PS sessions (3rd Brainmap)
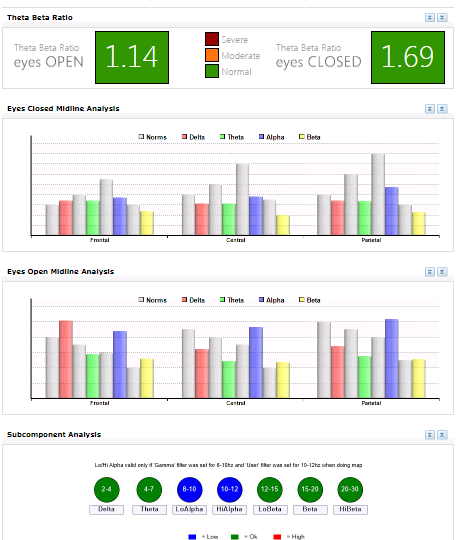
Figure 3. Pre-Intervention Midline analysis
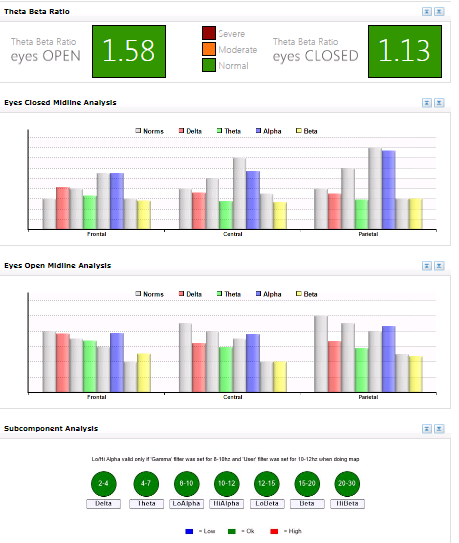
Figure 4. Post-Intervention Midline analysis
Discussion
PS training is purported to furnish broad therapeutic benefits. Initially, some subjects find photic stimulation to be disorienting while disentraining from dysregulated brainwave activity but by the sixth minute, most report experiencing hypnodissociative type effects and adaptive entrainment (even at non-targeted frequency bands) can be seen on EEG trend monitors. In this case in which the subject’s original QEEG was low-powered, presenting fast pulsating lights at 18 Hz seems to normalize brainwave frequencies in lower bandwidths, as well.
Defensive dissociation is a psychobiological state that protects individuals by separating them from overwhelming stress, but psychological detachment is not inherently pathological, particularly if prefrontal cortex activity is normalized. Dissociative cortical segregation renders memories unavailable and serves as an escape from over-arousal (Edge, 2001; Soffer‐Dudek, et al., 2019). Chronic or pathological dissociation causes fragmented and inadvertent shifts of awareness between disjointed experiences. Even transient dissociation in healthy people exposed to very low sensory stimulation attenuates awareness and memory functions (McKinnon, et al., 2016).
Healthy dissociation contrasts with pathological dissociation regarding controllability and flexibility of attentional processes and in terms of the anxiety that drives defensive or overwhelming dissociative experiences. Effective photic stimulation may untrain or disentrain rigid brain states of chronic anxiety or overarousal associated with addictions or even obsessive-compulsive disorder because attention can be diverted from rigidity and rumination while modulating awareness and enhancing sensitivity to experiences (Meyerson & Konichezky, 2011).
Conclusions:
Pulses of yellow light flashed in the eyes at 13.5 Hz in the left visual field and 18 Hz in the right visual field seemed to generate distributed effects across cortical brain regions and especially as observed in increased global alpha and posterior beta. The participant fell asleep more quickly with subsequent PS sessions and related that it could be partially attributed to the overall relaxing environment during PS exposure that she became accustomed to. She also expressed that she felt more alert after a few sessions and maintained these improvements throughout the intervention period. Since the participant repeatedly reported that not much had changed after the eighth session (between the wash-out period and the cessation of PS), it seemed to indicate an asymptotic limitation of additional normalization. This may indicate that PS effects are not very enduring after only 16 (24 minute) sessions, though. Even though the participant generally expressed that she experienced less anxiety, she also self-reported irritability, which may reflect some unmasking of fatigue or emerging efforts to adjust to higher arousal levels but is also known to occur during the process of overcoming dissociation. If there was a significant placebo effect, a rebound effect was not detected after the wash-out period or any time during the final eight sessions (based on observations, the participant’s self-reports, and comparisons between the 2nd QEEG and 3rd QEEG recordings). Administering differential CPS did not appear to induce abnormal cortical activity or abreactions.
The subject’s baseline QEEG recordings indicated low-powered brain maps. Therefore, fast flickering yellow lights were presented during PS sessions to entrain faster frequencies. The sessions seemed to induce meditative effects and normalizations in magnitude within alpha and beta (and to a lesser extent delta and theta) bandwidths. Since prestimulation magnitude levels were low, dramatic differences in alpha and beta magnitude may be partially attributable to a regression to the mean effect. Changes in one frequency (beta for instance), are known to have interactive effects in other frequencies (Rosenfeld, Reinhart, & Srivastava, 1997). Subjective preferences for yellow may also be (to some degree) the result of personal associations – an instance of individual-response specificity.
Identifying neural correlates of dissociation is beyond the scope of this paper. I am currently preparing a study with additional participants to examine potential neural correlates of state dissociation in response to presenting images of healthy and unhealthy food and mediating effects of photic stimulation
References
Adrian, E. D., & Matthews, B. H. (1934). The interpretation of potential waves in the cortex [PDF file]. The Journal of Physiology, 81(4), 440-471. https://physoc.onlinelibrary.wiley.com/doi/pdf/10.1113/jphysiol.1934.sp003147
Ali, M. R. (1972). Pattern of EEG recovery under photic stimulation by light of different colors. Clinical Neurophysiology, 33(3), 332-335. https://www.sciencedirect.com/science/article/abs/pii/0013469472901629
Alkozei, A., Smith, R., Pisner, D. A., Vanuk, J. R., Berryhill, S. M., Fridman, A., … & Killgore, W. D. (2016). Exposure to blue light increases subsequent functional activation of the prefrontal cortex during performance of a working memory task. Sleep, 39(9), 1671-1680. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4989256/?report=reader
Auerbach, E., Beller, A. J., Henkes, H. E., & Goldhaber, G. (1961). Electric potentials of retina and cortex of cats evoked by monocular and binocular photic stimulation. Vision Research, 1(1-2), 166-IN8. https://doi.org/10.1016/0042-6989(61)90027-X
Bajaj, S., Vanuk, J. R., Smith, R., Dailey, N. S., & Killgore, W. D. (2017). Blue-light therapy following mild traumatic brain injury: effects on white matter water diffusion in the brain. Frontiers in neurology, 8, 616. https://doi.org/10.3389/fneur.2017.00616
Beato, L., Rodríguez Cano, T., & Belmonte, A. (2003). Relationship of dissociative experiences to body shape concerns in eating disorders. European Eating Disorders Review: The Professional Journal of the Eating Disorders Association, 11(1), 38-45. https://doi.org/10.1002/erv.508
Berg, K., & Siever, D. (2009). A controlled comparison of audio-visual entrainment for treating Seasonal Affective Disorder. Journal of Neurotherapy, 13(3), 166-175. https://doi.org/10.1080/10874200903107314
Bob, P., Golla, M., Epstein, P. and Konopka, L., (2011). EEG complexity and attentional processes related to dissociative states. Clinical EEG and neuroscience, 42(3), pp.175-179. https://doi.org/10.1177/155005941104200306
Bonnefond, M., & Jensen, O. (2012). Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Current biology, 22(20), 1969-1974. https://doi.org/10.1016/j.cub.2012.08.029
Bowins, B. E. (2012). Therapeutic dissociation: Compartmentalization and absorption. Counselling Psychology Quarterly, 25(3), 307-317. https://doi.org/10.1080/09515070.2012.695278
Budzynski, T. H., Budzynski, H. K., Evans, J. R., & Abarbanel, A. (Eds.). (2009). Introduction to quantitative EEG and neurofeedback: Advanced theory and applications. Academic Press. https://books.google.com/books?id=PigKJuOSvbMC&pg=PA209&lpg=PA209&dq=%22dissociative+david+siever&source=bl&ots=AicfNASECp&sig=ACfU3U1rufc6NKTI6fAhjqwvDvrJCu0RQQ&hl=en&sa=X&ved=2ahUKEwj08eCe4uLoAhUPmuAKHUnpAJcQ6AEwA3oECAsQKQ#v=onepage&q=%22dissociative%20david%20siever&f=false
Budzynski, T., Jordy, J., Budzynski, H. K., Tang, H. Y., & Claypoole, K. (1999). Academic performance enhancement with photic stimulation and EDR feedback. Journal of Neurotherapy, 3(3-4), 11-21. https://doi.org/10.1300/J184v03n03_02
Carruthers, H. R., Morris, J., Tarrier, N., & Whorwell, P. J. (2010). The Manchester Color Wheel: development of a novel way of identifying color choice and its validation in healthy, anxious and depressed individuals. BMC medical research methodology, 10(1), 12. https://doi.org/10.1186/1471-2288-10-12
Carter, J. L., & Russell, H. L. (1993). A pilot investigation of auditory and visual entrainment of brain wave activity in learning disabled boys [PDF file]. Texas Researcher, 4(1), 65-75. http://www.neuromedicstechnology.com/Library/HR_TR.pdf
Collura, T., & Siever, D. (2008). Audio-visual entrainment in relation to mental health and EEG. In T. Budzynski, H. Budzynski, J. Evans, & A. Abarbanel (Eds.), Introduction to quantitative EEG and neurofeedback: Advanced theory and applications (2nd ed., pp. 193–223). Boston: Elsevier.
Cowan, R. L., Wood, J., Dietrich, M. S., de. B. Frederick, B., Lukas, S. E., & Renshaw, P. F. (2008). Differential effects of d‐amphetamine on red and blue light‐induced photic activation: A novel BOLD fMRI assay of human dopamine function. Synapse, 62(4), 268-272. https://doi.org/10.1002/syn.20491
Dalton, A. N., & Huang, L. (2013). Motivated forgetting in response to social identity threat. Journal of Consumer Research, 40(6), 1017-1038. https://doi.org/10.1086/674198
Diamond, M. J. (2020). Gazing Back, Playing Forward: Contemporary Psychoanalytic Musings on the Relational Essence of Hypnotherapeutic Action. American Journal of Clinical Hypnosis, 62(1-2), 12-30. https://doi.org/10.1080/00029157.2019.1580558
Dickie, E. W., Brunet, A., Akerib, V., & Armony, J. L. (2011). Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia, 49(7), 1771-1778. https://doi.org/10.1016/j.neuropsychologia.2011.02.055
Dijk, D. J., & Archer, S. N. (2009). Light, sleep, and circadian rhythms: together again. PLoS biology, 7(6), e1000145. https://doi.org/10.1371/journal.pbio.1000145
Edge, L. W. (2001). The spectrum of dissociation: From pathology to self-realization [PDF File]. Journal of Transpersonal Psychology, 33(1), 53-63. http://atpweb.org/jtparchive/trps-33-01-01-053.pdf
Edge, L. W. (2004). A phenomenological study of directed dissociation [PDF file]. Journal of Humanistic Psychology, 44(2), 155-181. https://journals.sagepub.com/doi/pdf/10.1177/0022167804263025?casa_token=2tXZJdySQ_gAAAAA:J_4Oj7ADGwGWLZXbjHXUw7PtgJIOj_YOsCsHna96TVsi3MgX8W9PL5qfNqyHTT9aPEeMzb51mmseWw
Figueiro, M. G., Bierman, A., & Rea, M. S. (2013). A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nature and science of sleep, 5, 133. https://doi.org/10.2147/NSS.S52203
Fisher, J. (2001, May). Dissociative phenomena in the everyday lives of trauma survivors. In Boston University Medical School Psychological Trauma Conference: May. http://www.tricountywomenscentre.org/uploads/5/7/6/6/5766610/fisher_-_dissociative_phenomena_in_the_everyday_lives_of_trauma_survivors.pdf
Fisher, J. (2014). The treatment of structural dissociation in chronically traumatized patients. Trauma treatment in practice: Complex trauma and dissociation.
Fox, P. T., & Raichle, M. E. (1985). Stimulus rate determines regional brain blood flow in striate cortex [PDF file]. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 17(3), 303-305. http://mylaxman.fr/download/studies/Stimulus%20Rate%20Determines%20Regional%20Brain%20Blood%20Flow%20in%20Striate%20Cortex%20blood%20flow%20induced%20by%20two%20modes%20of%20photic%20stimuli.pdf
Frederick, J., Lubar, J., Rasey, H., Brim, S., & Blackburn, J. (1999). Effects of 18.5 Hz audiovisual stimulation on EEG amplitude at the vertex. Applied Psychophysiology and Biofeedback, 24(2), 120–121. https://doi.org/10.1300/J184v03n03_03
Geerdink, M., Walbeek, T. J., Beersma, D. G., Hommes, V., & Gordijn, M. C. (2016). Short blue light pulses (30 min) in the morning support a sleep-advancing protocol in a home setting. Journal of biological rhythms, 31(5), 483-497. https://doi.org/10.1177/0748730416657462
Giesbrecht, T., Jongen, E. M., Smulders, F. T., & Merckelbach, H. (2006). Dissociation, resting EEG, and subjective sleep experiences in undergraduates [PDF file]. The Journal of nervous and mental disease, 194(5), 362-368. http://haraldmerckelbach.nl/artikelen_engels/2006/Dissociation,%20Resting%20EEG,%20And%20Subjective%20Sleep%20Experiences%20In%20Undergraduates.pdf
Gruzelier, J. (2009). A theory of alpha/theta neurofeedback, creative performance enhancement, long distance functional connectivity and psychological integration. Cognitive processing, 10(1), 101-109. https://doi.org/10.1007/s10339-008-0248-5
Hanslmayr, S., Gross, J., Klimesch, W., & Shapiro, K. L. (2011). The role of alpha oscillations in temporal attention. Brain research reviews, 67(1-2), 331-343. https://doi.org/10.1016/j.brainresrev.2011.04.002
Heinrichs-Graham, E., & Wilson, T. W. (2012). Presence of strong harmonics during visual entrainment: a magnetoencephalography study. Biological psychology, 91(1), 59-64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3407313/
Hiroshi, K. (1987). Studies on the Temporal Frequency Characteristics of Vision by Photic Driving Method (III): Temporal Frequency Characteristics of Color Vision. Tohoku Psychologica Folia, 46(1-4), 1-12. https://scholar.googleusercontent.com/scholar?q=cache:6bVQuCa3kb8J:scholar.google.com/+STUDIES+ON+THE+TEMPORAL+FREQUENCY+CHARACTERISTICS+OF+VISION+BY+PHOTIC+DRIVING+METHOD+(III)+&hl=en&as_sdt=0,10
Jensen, O., & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Frontiers in human neuroscience, 4, 186. https://doi.org/10.3389/fnhum.2010.00186
Jokić-Begić, N., & Begić, D. (2003). Quantitative electroencephalogram (qEEG) in combat veterans with post-traumatic stress disorder (PTSD). Nordic Journal of Psychiatry, 57(5), 351-355. https://doi.org/10.1080/08039480310002688
Joyce, M., & Siever, D. (2000). Audio-visual entrainment program as a treatment for behavior disorders in a school setting. Journal of Neurotherapy, 4(2), 9-25. https://doi.org/10.1300/J184v04n02_04
Kroger, W.S., & Schneider, S. A. (1959) An electronic aid for hypnotic induction: A preliminary report. [Abstract]. International Journal of Clinical and Experimental Hypnosis, 7, 93-98. https://psycnet.apa.org/record/1961-00924-001
Kunst, J. R., & Hohle, S. M. (2016). Meat eaters by dissociation: How we present, prepare and talk about meat increases willingness to eat meat by reducing empathy and disgust. Appetite, 105, 758-774. https://doi.org/10.1016/j.appet.2016.07.009
Lack, L., & Wright, H. (1993). The effect of evening bright light in delaying the circadian rhythms and lengthening the sleep of early morning awakening insomniacs. Sleep, 16(5), 436-443. https://doi.org/10.1093/sleep/16.5.436
La Mela, C., Maglietta, M., Castellini, G., Amoroso, L., & Lucarelli, S. (2010). Dissociation in eating disorders: relationship between dissociative experiences and binge-eating episodes. Comprehensive Psychiatry, 51(4), 393-400. https://doi.org/10.1016/j.comppsych.2009.09.008
Lazarev, V. V., Infantosi, A. F. C., Valencio-de-Campos, D., & de Azevedo, L. C. (2004). Topographic aspects of photic driving in the electroencephalogram of children and adolescents. Brazilian journal of medical and biological research, 37(6), 879-891. http://dx.doi.org/10.1590/S0100-879X2004000600014
Lazarev, V. V., Simpson, D. M., Schubsky, B. M., & Deazevedo, L. C. (2001). Photic driving in the electroencephalogram of children and adolescents: harmonic structure and relation to the resting state. Brazilian Journal of Medical and Biological Research, 34(12), 1573-1584. http://dx.doi.org/10.1590/S0100-879X2001001200010
Leonard, K. N., Telch, M. J., & Harrington, P. J. (1999). Dissociation in the laboratory: A comparison of strategies [PDF file]. Behaviour research and therapy, 37(1), 49-61. https://s3.amazonaws.com/academia.edu.documents/36225614/Leonard1999.pdf?response-content-disposition=inline%3B%20filename%3DDissociation_in_the_laboratory_a_compari.pdf&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIAIWOWYYGZ2Y53UL3A%2F20191130%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Date=20191130T041215Z&X-Amz-Expires=3600&X-Amz-SignedHeaders=host&X-Amz-Signature=0837e922e1b74707948846aa1f7e2b88edd18893003e7ee34a990c6c4fa736d0
Lüscher, M. (1971). The Luscher Color Test. New York, NY: Random House, Inc.
Lynch, J. J., & Paskewitz, D. A. (1979). On the mechanisms of the feedback control of human brain-wave activity. In Mind/Body Integration (pp. 325-340). Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-2898-8_28
McKinnon, M. C., Boyd, J. E., Frewen, P. A., Lanius, U. F., Jetly, R., Richardson, J. D., &
Lanius, R. A. (2016). A review of the relation between dissociation, memory, executive functioning and social cognition in military members and civilians with neuropsychiatric conditions. Neuropsychologia, 90, 210-234. https://doi.org/10.1016/j.neuropsychologia.2016.07.017
McShane, J. M., & Zirkel, S. (2008). Dissociation in the binge–purge cycle of bulimia nervosa. Journal of Trauma & Dissociation, 9(4), 463-479. https://doi.org/10.1080/15299730802225680
Meeuwissen, E. B., Takashima, A., Fernández, G., & Jensen, O. (2011). Increase in posterior alpha activity during rehearsal predicts successful long‐term memory formation of word sequences. Human brain mapping, 32(12), 2045-2053. https://doi.org/10.1002/hbm.21167
Mentis, M. J., Alexander, G. E., Grady, C. L., Horwitz, B., Krasuski, J., Pietrini, P., & Rapoport, S. I. (1997). Frequency variation of a pattern-flash visual stimulus during PET differentially activates brain from striate through frontal cortex. Neuroimage, 5(2), 116-128. https://doi.org/10.1006/nimg.1997.0256
Merckelbach, H., Muris, P., Horselenberg, R., & Stougie, S. (2000). Dissociative experiences, response bias, and fantasy proneness in college students. Personality and Individual Differences, 28(1), 49-58. https://doi.org/10.1016/S0191-8869(99)00079-3
Meyerson, J., & Konichezky, A. (2011). Hypnotically induced dissociation (HID) as a strategic intervention for enhancing OCD treatment. American Journal of Clinical Hypnosis, 53(3), 169-181. https://doi.org/10.1080/00029157.2011.10401755
Miskovic, V., & Keil, A. (2015). Reliability of event-related EEG functional connectivity during visual entrainment: Magnitude squared coherence and phase synchrony estimates. Psychophysiology, 52(1), 81–89. https://doi.org/10.1111/psyp.12287
Münch, M., Plomp, G., Thunell, E., Kawasaki, A., Scartezzini, J. L., & Herzog, M. H. (2014).Different colors of light lead to different adaptation and activation as determined by high-density EEG [PDF file]. NeuroImage, 101, 547-554. https://s3.amazonaws.com/academia.edu.documents/39296393/5493d4c80cf286fe31269570.pdf?response-content-disposition=inline%3B%20filename%3DDifferent_colors_of_light_lead_to_differ.pdf&X-Amz-Algorithm=AWS4-HMAC-SHA256&X-Amz-Credential=AKIAIWOWYYGZ2Y53UL3A%2F20190614%2Fus-east-1%2Fs3%2Faws4_request&X-Amz-Date=20190614T060709Z&X-Amz-Expires=3600&X-Amz-SignedHeaders=host&X-Amz-Signature=6b95c35ba881753996430330b363a6c0a246be64b8ca1c9129fd26fa45e96d4a
Nolan, R. F., Dai, Y., & Stanley, P. D. (1995). An investigation of the relationship between color choice and depression measured by the Beck Depression Inventory. Perceptual and motor skills, 81(3_suppl), 1195-1200. https://doi.org/10.2466/pms.1995.81.3f.1195
Novembre, G., Sammler, D., & Keller, P. E. (2016). Neural alpha oscillations index the balance between self-other integration and segregation in real-time joint action. Neuropsychologia, 89, 414-425. ://doi.org/10.1016/j.neuropsychologia.2016.07.027
Peebles, M. J. (2018). Harm in hypnosis: Three understandings from psychoanalysis that can help [PDF file]. American Journal of Clinical Hypnosis, 60(3), 239-261. https://www.tandfonline.com/doi/pdf/10.1080/00029157.2018.1400811?casa_token=solb6XIWZuwAAAAA:zYdgsWQSBc3PLKcADvzPfb3NV7SZY3r5gxkFVdPHA1je1_9vaHCY8sdOEB2khzIJwclq_UXYTpYzNQ
Phipps-Nelson, J., Redman, J. R., Schlangen, L. J., & Rajaratnam, S. M. (2009). Blue light exposure reduces objective measures of sleepiness during prolonged nighttime performance testing. Chronobiology International, 26(5), 891-912. https://www.tandfonline.com/doi/full/10.1080/07420520903044364?casa_token=_3PCwDQsm2YAAAAA:WeEs7IeB19veGMy2RFC9uaDtdy4Zta65Ud4TsXpgSFPa2rLe9V5lmIfcyNWsKcOx4sIMv6GJC_DdOw
Rabellino, D., Burin, D., Harricharan, S., Lloyd, C., Frewen, P. A., McKinnon, M. C., & Lanius, R. A. (2018). Altered sense of body ownership and agency in posttraumatic stress disorder and its dissociative subtype: a rubber hand illusion study. Frontiers in human neuroscience, 12, 163. https://doi.org/10.3389/fnhum.2018.00163
Rahimi, M., Makarem, J., & Rooyan, P. (2013). Effects of a flash of light in different colors on venous cannulation pain: a randomized, controlled trial. Journal of clinical anesthesia, 25(1), 42-46. https://doi.org/10.1016/j.jclinane.2012.06.006
Roberts, B. M., Clarke, A., Addante, R. J., & Ranganath, C. (2018). Entrainment enhances theta oscillations and improves episodic memory. Cognitive neuroscience, 9(3-4), 181-193. https://doi.org/10.1080/17588928.2018.1521386
Roberts, J. A., & Robinson, P. A. (2012). Quantitative theory of driven nonlinear brain dynamics. Neuroimage, 62(3), 1947-1955. https://doi.org/10.1016/j.neuroimage.2012.05.054
Roohi-Azizi, M., Azimi, L., Heysieattalab, S., & Aamidfar, M. (2017). Changes of the brain’s bioelectrical activity in cognition, consciousness, and some mental disorders. Medical journal of the Islamic Republic of Iran, 31, 53. https://doi.org/10.14196/mjiri.31.53
Rosenfeld, J. P., Reinhart, A. M., & Srivastava, S. (1997). The effects of alpha (10-Hz) and beta (22-Hz)“entrainment” stimulation on the alpha and beta EEG bands: individual differences are critical to prediction of effects. Applied Psychophysiology and Biofeedback, 22(1), 3-20. https://doi.org/10.1023/A:1026233624772
Rothgerber, H. (2019). Meat-related cognitive dissonance: A conceptual framework for understanding how meat eaters reduce negative arousal from eating animals. Appetite, 146, 104511. https://doi.org/10.1016/j.appet.2019.104511
Ruiman, X., Jingli, Y., & Zhen, Q. (1997). The clinical and SPECT studies of photic stimulation therapy in patients with homonymous hemianopia [J]. Chinese Journal of Rehabilitation Theory & Practice, 3. http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZKLS199703002.htm
Russ, M. J., Campbell, S. S., Kakuma, T., Harrison, K., & Zanine, E. (1999). EEG theta activity and pain insensitivity in self-injurious borderline patients. Psychiatry Research, 89(3), 201-214. https://doi.org/10.1016/S0165-1781(99)00113-4
Sappey-Marinier, D., Calabrese, G., Fein, G., Hugg, J. W., Biggins, C., & Weiner, M. W. (1992). Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy [PDF File]. Journal of Cerebral Blood Flow & Metabolism, 12(4), 584-592. https://journals.sagepub.com/doi/pdf/10.1038/jcbfm.1992.82
Sierra, M., & Berrios, G. E. (1998). Depersonalization: neurobiological perspectives. Biological psychiatry, 44(9), 898-908. https://doi.org/10.1016/S0006-3223(98)00015-8
Siever, D. (2002). Audio-Visual Entrainment as a Treatment Modality for Post-Traumatic StressDisorder. https://bio-medical.com/media/support/david_ptsd_study.pdf
Siever, D. (2018, December 15). Neurotechnology Forever Transformed My Life. [Video File].
Siever, D. (2014, November 22). Physiology and Clinical Applications of Audio-visual Entrainment Technology. International Light Association [Video file]. https://www.youtube.com/watch?v=yNFyBo3gIsM
Siever, D. (2016, July 11). Physiology and Clinical Applications of Audio-visual Entrainment Technology. International Light Association [Video file]. https://www.youtube.com/watch?v=OBE0fRkDFws
Siever, D. (2015). Stimulation Technologies:” New” Trends in” Old” Techniques. Biofeedback, 43(4), 180-192. https://doi.org/10.5298/1081-5937-43.04.11
Soffer‐Dudek, N., Todder, D., Shelef, L., Deutsch, I., & Gordon, S. (2019). A neural correlate for common trait dissociation: Decreased EEG connectivity is related to dissociative absorption [PDF file]. Journal of personality, 87(2), 295-309. https://onlinelibrary.wiley.com/doi/pdf/10.1111/jopy.12391?
Spitzer, C., Barnow, S., Freyberger, H. J., & Grabe, H. J. (2006). Recent developments in the theory of dissociation. World Psychiatry, 5(2), 82. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1525127/
Takahashi, T., & Tsukahara, Y. (1976). Influence of color on the photoconvulsive response. Electroencephalography and Clinical Neurophysiology, 41(2), 124-136. https://scholar.google.com/scholarstart=30&q=photic+stimulation+yellow+light+&hl=en&as_sdt=0,10#d=gs_qabs&u=%23p%3DJLtpQSmznJsJ
Tamarova, Z. A., Limansky, Y., & Gulyar, S. A. (2009). Antinociceptive effects of colorpolarized light in animal with formalin test [PDF file]. Fiziol. J, 3, 81-93. http://biph.kiev.ua/fiziol/2009_V.55/Fiziologichnyi%20Zhurnal%2055(3)_2009/Fiziologichnyi%20Zhurnal%2055(3)_2009_81-93.pdf
Tang, H. Y. J., Riegel, B., McCurry, S. M., & Vitiello, M. V. (2016). Open-loop audio-visualstimulation (AVS): A useful tool for management of insomnia?. Applied psychophysiology and biofeedback, 41(1), 39-46. https://doi.org/10.1007/s10484-015-9308-7
Tang, H. Y. J., Vitiello, M. V., Perlis, M., & Riegel, B. (2015). Open-loop neurofeedbackaudiovisual stimulation: A pilot study of its potential for sleep induction in older adults. Applied psychophysiology and biofeedback, 40(3), 183-188. https://doi.org/10.1007/s1048
van der Kolk, B. A., Hodgdon, H., Gapen, M., Musicaro, R., Suvak, M. K., Hamlin, E., &Spinazzola, J. (2016). A randomized controlled study of neurofeedback for chronic PTSD. PloS one, 11(12), e0166752. https://doi.org/10.1371/journal.pone.0166752
Vazquez, S. R. (2005, Summer). A new paradigm for PTSD treatment: Emotional
Transformation Therapy. Annals of the American Psychotherapy Association, 8(2), 18+. Retrieved from https://link-gale-com.dax.lib.unf.edu/apps/doc/A134955705/AONE?u=jack91990&sid=AONE&xid=76545d45
Vermetten, E., Schmahl, C., Lindner, S., Loewenstein, R. J., & Bremner, J. D. (2006). Hippocampal and amygdalar volumes in dissociative identity disorder. American Journal of Psychiatry, 163(4), 630-636. https://doi.org/10.1176/appi.ajp.163.4.630
Waller, G., Ohanian, V., Meyer, C., Everill, J., & Rouse, H. (2001). The utility of dimensional and categorical approaches to understanding dissociation in the eating disorders. British Journal of Clinical Psychology, 40(4), 387-397. https://doi.org/10.1348/014466501163878
Yatsenko, M. V., Kaigorodova, N. Z., Prokhorov, N. V., & Rastyagaeva, O. V. (2015). Influenceof color stimulation on situational anxiety of first-year students. 2015 International Conference on Biomedical Engineering & Computational Technologies (SIBIRCON), 55. Retrieved from https://login.dax.lib.unf.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=edb&AN=112645124&site=eds-live&scope=site
Zhang, Y., Wang, F., Luo, X., Wang, L., Sun, P., Wang, M., … & Sugai, T. (2015). CognitiveImprovement by Photic Stimulation in a Mouse Model of Alzheimer’s Disease. CurrentAlzheimer Research, 12(9), 860-869.
https://www.ingentaconnect.com/content/ben/car/2015/00000012/00000009/art00006